1. Background
Involving approximately 2.8% of the general population worldwide, hepatitis C virus (HCV) infection is one of the most important public health concerns, which is estimated to affect 185 million people (1). A remarkable number of complications such as liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) have been recounted for chronic HCV infection (2). The majority of cases with HCV infection remain undiagnosed because of the asymptomatic nature of the disease (3). Nevertheless, nearly all of hepatitis C complications are preventable by treatment if the disease is diagnosed at early phases (4). Approximately, 30% of patients with chronic hepatitis C (CHC) infection have HCV genotype-2 (HCV-2) or -3 (5).
Newly proposed medications for HCV infection have increased the possibility of successful therapy and have provided a wide range of choices for treatment. Various oral and injection medications have been approved for different HCV genotypes. The main goal of these treatments is to achieve a sustained virological response (SVR), which has made regimens with direct-acting antiviral agents (DAAs) superior to traditional pegylated-interferon (PegIFN) and ribavirin (RBV) combination therapy (6). The rate of SVR to PegIFN and RBV combination therapy in patients with HCV-2 or -3 infections has been reported as 60% - 90% (7-11). The combination of Sofosbuvir (SOF) and RBV with or without PegIFN is being prescribed for the treatment of patients with HCV-2 or -3 and can lead to 60% to > 90% SVR (12-14). However, there is not a concise conclusion on the preference of each regimen. Therefore, the need for a review of the abundant relevant studies comes up to finally determine which regimen should be prescribed for each HCV genotype.
2. Objectives
In this systematic review and meta-analysis, we aimed to evaluate the efficacy of regimens of SOF plus RBV with or without PegIFN for the treatment of patients with HCV-2 or -3 infections.
3. Methods
3.1. Data Resources and Search Strategies
We systematically searched electronic databases including PubMed, Scopus, ScienceDirect, and Web of Science. We appropriately combined keywords of “Sofosbuvir”, “Sovaldi”, “GS-7977”, “Pegylated-interferon”, and “Ribavirin” for use in different databases (Appendix 1 in Supplementary File). Initially, our search was performed in September 2015 and we updated it in September 2016. The search language was restricted to English. In addition, for identifying any additional studies, we screened the reference lists of the ultimately included studies in this survey.
3.2. Eligibility Criteria
We included any cohort or clinical trial that evaluated the effect of 12- or 24-week therapy with SOF + RBV or SOF + RBV + PegIFN on the SVR rate of patients with HCV-2 or -3 infections. Patients co-infected with HBV or HIV and those with a history of kidney or liver transplantation, a history of treatment with DAAs, and decompensated cirrhosis (Child-Pugh B and C) were not considered for inclusion. All studies reporting SVR with the inclusion of more than 10 patients were included in the quantitative analysis; otherwise, they were excluded for the final quantitative analysis.
3.3. Study Selection, Quality Assessment, and Data Extraction
Two authors (M.E.B and M.H.K) independently investigated all identified papers through database searching and screening in different levels including title, abstract, and full-text. The screening process was completely based on the PRISMA guideline for reporting of systematic reviews (15). Any disagreements between the authors were resolved by neutral discussion. Cochrane’s tool for risk of bias assessment (16) and Newcastle Ottawa scale (17) were applied for the evaluation of clinical trial and cohort studies, respectively. Data regarding publication details and study patients in each paper were extracted, including first author name, publication year, sample size, patients’ age, gender, baseline HCV RNA level, history of previous treatment, cirrhosis, and rate of SVR.
3.4. Data Analysis
We used STATA 11 for all statistical analyses in this survey. Based on the result of the test for heterogeneity (chi-squared and I-squared), fixed- or random-effects models were used with command “metaprop” for the calculation of the pooled SVR rate and 95% confidence interval (CI). In addition, Begg’s and Egger’s tests were used for the publication bias assessment.
4. Results
4.1. Study Screening
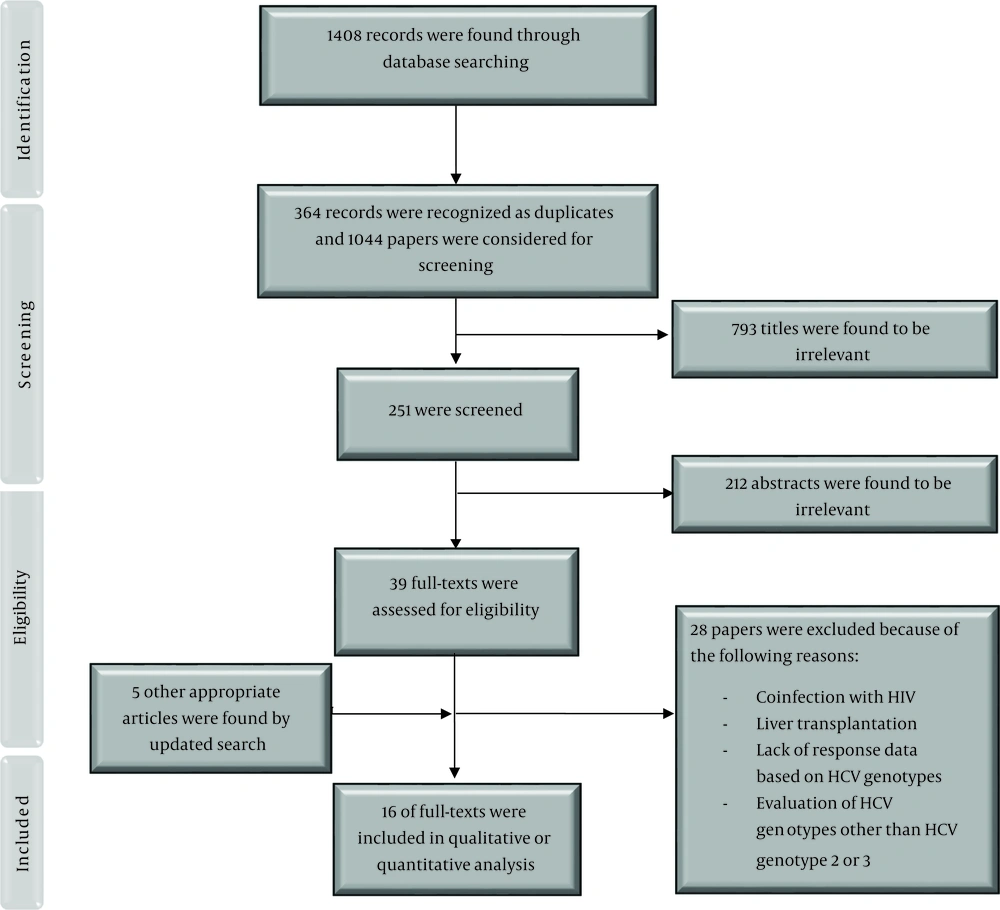
We found 1408 records through database searching and after removing duplicates, 1044 papers were investigated. We excluded 793 articles by title screening and 212 by abstract screening. Finally, together with the updated search, 16 papers were included in this study (Figure 1).
4.2. Risk of Bias Assessment
The results of quality assessment for the included clinical trial and cohort studies are shown in Tables 1 and 2, respectively. All clinical trials and cohort studies were categorized as low risk and high quality, respectively. No study was excluded based on this assessment.
| First Author (Reference) | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participant and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition) | Selective Reporting (Reporting Bias) | Co-interventions | Intention to Treat Analysis | Group Similarity at Baseline | Compliance | Timing of Outcome Assessments | Other Biases | Score | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foster, G. R. (18) | - | - | + | + | - | - | - | - | - | - | - | - | 10 | Low |
| Zeuzem, S. (19) | ? | - | + | + | - | - | - | - | - | - | - | - | 9 | Low |
| Lawitz, E. (20) | ? | + | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Gane, J. E. (21) | ? | - | + | + | - | - | - | - | - | - | - | - | 9 | Low |
| Jacobson, I. M. (12) | ? | ? | - | ? | - | - | - | - | - | - | - | - | 9 | Low |
| Lawitz, E. (22) | + | + | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Omata, M. (14) | + | + | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Ahn, S. H. (23) | + | + | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Kao, J.H. (13) | + | + | + | + | - | - | - | - | - | - | - | - | 8 | Low |
Risk of Bias Assessment for the Included Clinical Trial Studies
Quality Assessment of Cohort Studies Based on the New Castle Ottawa Scale
4.3. Characteristics of the Included Studies
The included studies were clinical trials and cohort studies published since 2012. The characteristics of the studies on the treatment of patients with HCV genotype-2 or -3 are shown in Tables 3 and 4, respectively.
| First Author (Reference) | Treatment History | Publication Year | Sample Size | Mean Age (SD or Range) | Male Gender (%) | Treatment Regimen, Duration (Week) | Mean HCV RNA Level (SD or Range) | Cirrhosis (%) |
|---|---|---|---|---|---|---|---|---|
| Steinebrunner, N. (25) | Mix | 2015 | 14 | 50 ± 12 | ND | SOF + RBV, 12 | 3.23 ± 6.61 | 42 |
| Omata, M. (14) | Mix | 2014 | 153 | 57 | 54 | SOF + RBV, 12 | 6.3 ± 0.84 | 11 |
| Gane, J. E. (21) | ND | 2013 | 4 | 47 | ND | SOF + RBV, 12 | 6.7 (5.7 - 7.1) | ND |
| Lawitz, E. (20) | TN | 2013 | 70 | 48 (20 - 72) | 67 | SOF + RBV, 12 | 6.0 ± 0.8 | ND |
| Jacobson, I. M. (12) | TN | 2013 | 109 | 52 (21 - 75) | ND | SOF + RBV, 12 | 6.3 ± 0.77 | ND |
| Zeuzem, S. (19) | Mix | 2014 | 73 | 58 (28 - 74) | 55 | SOF + RBV, 12 | 6.5 ± 0.7 | 15 |
| Welzel, T. M. (27) | Mix | 2016 | 283 | 59 (21 - 80) | 61.5 | SOF + RBV, 12 | 6.3 | 21.9 |
| Ahn, S. H. (23) | Mix | 2016 | 129 | 55 | 45 | SOF + RBV, 12 | 6 ± 1 | 10 |
| Kao, J-H. (13) | Mix | 2016 | 87 | 53 | 41 | SOF + RBV, 12 | 6.4 ± 0.91 | 15 |
| Cho, Y. (28) | ND | 2015 | 6 | 52 | 50 | SOF + RBV, 12 | 6.2 | 50 |
| Backus, L. I. (29) | Mix | 2015 | 619 | 60.9 ± 7.0 | 96 | SOF + RBV, 12 | 6.2 ± 0.8 | ND |
| Massoumy, B. (24) | Mix | 2016 | 32 | 53.5 | 53 | SOF + RBV, 12 | 6.08 (1.18 - 7.78) | 31.2 |
| Jayasekera, C. R. (26) | Mix | 2015 | 16 | 62.8 | 43 | SOF + RBV, 12 | ND | 31.2 |
| Tacke, F. (30) | Mix | 2016 | 191 | 53 (19 - 85) | 62 | SOF + RBV, 12 | ND | 19 |
| Foster, G. R. (18) | TE | 2015 | 16 | 50 ± 10.2 | 68 | SOF + RBV + PegIFN, 12 | 6.3 ± 0.69 | 100 |
| Lawitz, E. (22) | TE | 2015 | 23 | 58 | 61 | SOF + RBV + PegIFN, 12 | 6.4 ± 0.7 | 61 |
| Gane, J. E. (21) | ND | 2013 | 4 | 46 (37 - 57) | 82 | SOF + RBV + PegIFN, 12 | 6.5 (5.1 - 7.3) | 0 |
Characteristics of the Included Studies on the Treatment of Patients with HCV Genotype-2 Infection (Appropriate Arms and Groups in Each Study of the Treatment of HCV-Infected Patients with Sofosbuvir Plus Ribavirin with or without Pegylated-Interferon)
| First Author (Reference) | Treatment History | Publication Year | Sample Size | Mean Age (SD or Range) | Male Gender (%) | Treatment regimen, Duration (week) | Mean HCV RNA Level (SD or Range) | Cirrhosis (%) |
|---|---|---|---|---|---|---|---|---|
| Zeuzem, S. (19) | Mix | 2013 | 11 | 46 | 55 | SOF + RBV, 12 | 6.2 ± 0.8 | 18 |
| Jacobson, I. M. (12) | ND | 2012 | 98 | 52 | 57 | SOF + RBV, 12 | 6.3 ± 0.77 | 21 |
| Lawitz, E. (20) | ND | 2012 | 183 | 48 (20 - 72) | 67 | SOF + RBV, 12 | 6.0 ± 0.8 | ND |
| Jayasekera, C. R. (26) | Mix | 2015 | 6 | 62 | 50 | SOF + RBV, 24 | ND | 33.3 |
| Maasoumy, B. (24) | Mix | 2016 | 33 | 50 | 67 | SOF + RBV, 24 | 6.08 | 47.6 |
| Foster, G. R. (18) | Mix | 2015 | 182 | 49 ± 9.8 | 65 | SOF + RBV, 24 | 6.2 ± 0.71 | 37 |
| Zeuzem, S. (19) | Mix | 2013 | 250 | 48 (19 - 69) | 62 | SOF + RBV, 24 | 6.3 ± 0.7 | 24 |
| Massoumy, B. (24) | Mix | 2016 | 16 | 49.5 | 62.5 | SOF + RBV + PegIFN, 12 | 5.94 | 37.5 |
| Foster, G. R. (18) | Mix | 2015 | 181 | 50 ± 10.2 | 67 | SOF + RBV + PegIFN, 12 | 6.3 ± 0.69 | 38 |
| Lawitz, E. (22) | TE | 2013 | 24 | 54 (39 - 64) | 75 | SOF + RBV + PegIFN, 12 | 6.0 ± 0.6 | 50 |
| Gane, J. E. (21) | ND | 2013 | 7 | 46 (37 - 57) | 82 | SOF + RBV + PegIFN, 12 | 6.5 (5.1 - 7.3) | 0 |
Characteristics of the Included Studies on the Treatment of Patients with HCV Genotype-3 Infection (Appropriate Arms and Groups in Each Study of the Treatment of HCV-Infected Patients with Sofosbuvir Plus Ribavirin with or without Pegylated-Interferon)
4.4. Outcome Evaluation
We evaluated two regimens for the treatment of patients with HCV-2 infection including SOF + RBV for 12 weeks and SOF + RBV + PegIFN for 12 weeks and three regimens for HCV-3 infection including SOF + RBV for 12 weeks, SOF + RBV for 24 weeks, and SOF + RBV + PegIFN for 12 weeks. A summary of the results of the meta-analysis of these regimens is shown in Table 5.
| HCV Genotype | Treatment Regimen | Treatment Duration, Week | Number of Included Studies, n | Sample Size, n | SVR, % | Lower CI of SVR, % | Upper CI of SVR, % |
|---|---|---|---|---|---|---|---|
| HCV-2 | SOF + RBV | 12 | 12 | 1776 | 92 | 87 | 96 |
| HCV-2 | SOF + RBV + PegIFN | 12 | 2 | 39 | 95 | 85 | 100 |
| HCV-3 | SOF + RBV | 12 | 3 | 292 | 55 | 44 | 66 |
| HCV-3 | SOF + RBV | 24 | 3 | 465 | 81 | 72 | 88 |
| HCV-3 | SOF + RBV + PegIFN | 12 | 3 | 221 | 93 | 85 | 99 |
A Summary of the Results of Meta-Analysis of the Evaluated Treatment Regimens
4.4.1. Treatment of HCV Genotype-2 with Sofosbuvir Plus Ribavirin for 12 Weeks
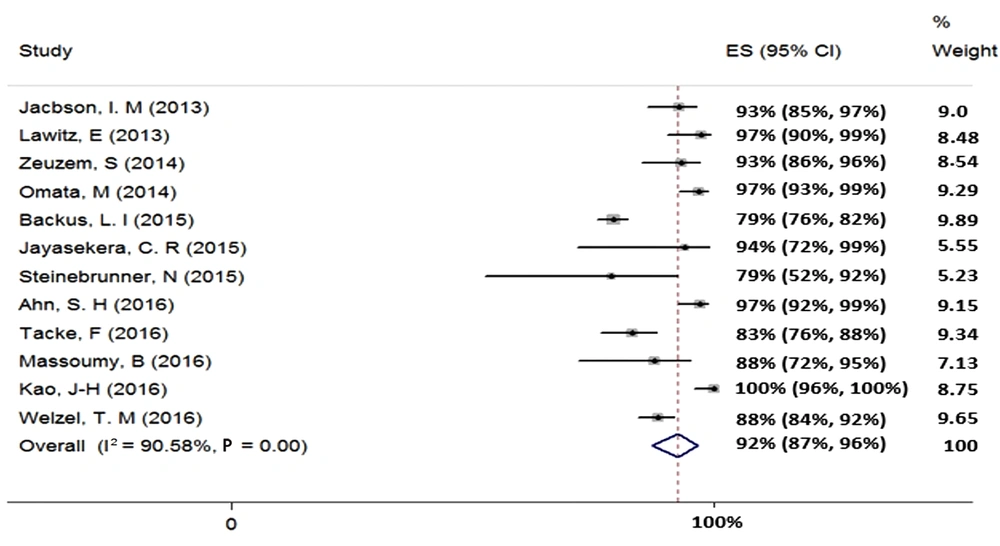
We found 12 studies (total sample size = 1776) evaluating the effect of 12-week treatment with SOF + RBV on patients with HCV-2 infection. Because of the existence of heterogeneity between the results of the included studies (χ2 = 116.82, P = 0.00, I2 = 90.58%), we used a random-effects model that calculated the pooled SVR rate (95% CI) as 92% (87% - 96%) (Figure 2). The publication bias was assessed based on Begg’s (P = 0.89) and Egger’s (P = 0.05) tests and showed a considerable bias.
4.4.2. Treatment of HCV Genotype-2 with Sofosbuvir Plus Ribavirin and Pegylated-Interferon for 12 Weeks
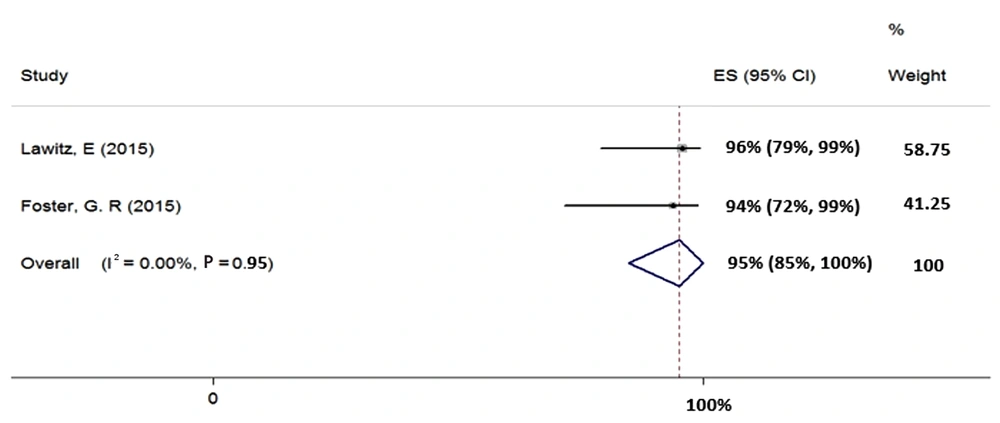
We included two studies in the meta-analysis of 12-week treatment with SOF + RBV + PegIFN in patients with HCV-2 (total sample size = 39). Since the heterogeneity was not seen between the results of these two studies (χ2 = 0.0, P = 0.95, I2 = 0.0%), we applied a fixed-effects model that calculated the pooled SVR rate (95% CI) as 95% (85% - 100%) (Figure 3).
4.4.3. Treatment of HCV Genotype-3 with Sofosbuvir Plus Ribavirin for 12 Weeks
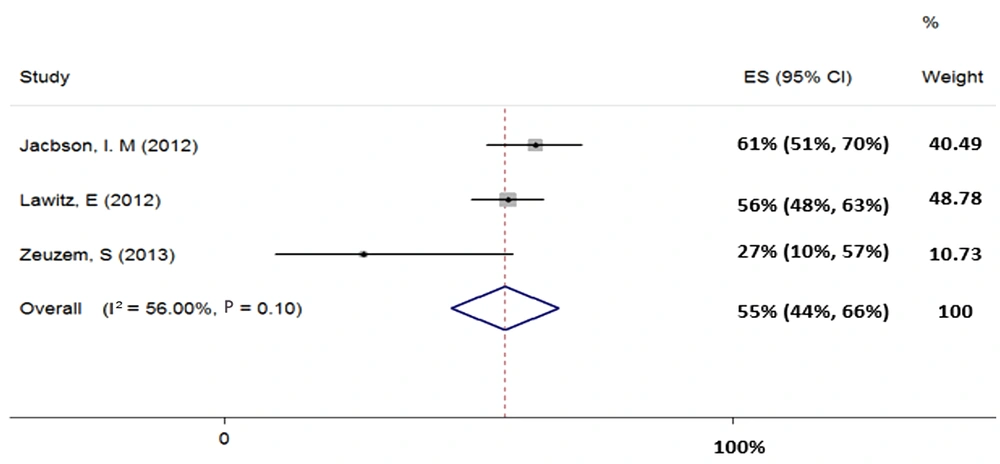
Three studies (total sample size = 292) were evaluated for the regimen of SOF + RBV in patients with HCV-3. Our results showed heterogeneity (χ2 = 4.55, P = 0.10, I2 = 56%) and based on random-effects model, the pooled SVR rate (95% CI) was calculated as 55% (44% - 66%) (Figure 4).
4.4.4. Treatment of HCV Genotype-3 with Sofosbuvir Plus Ribavirin for 24 Weeks
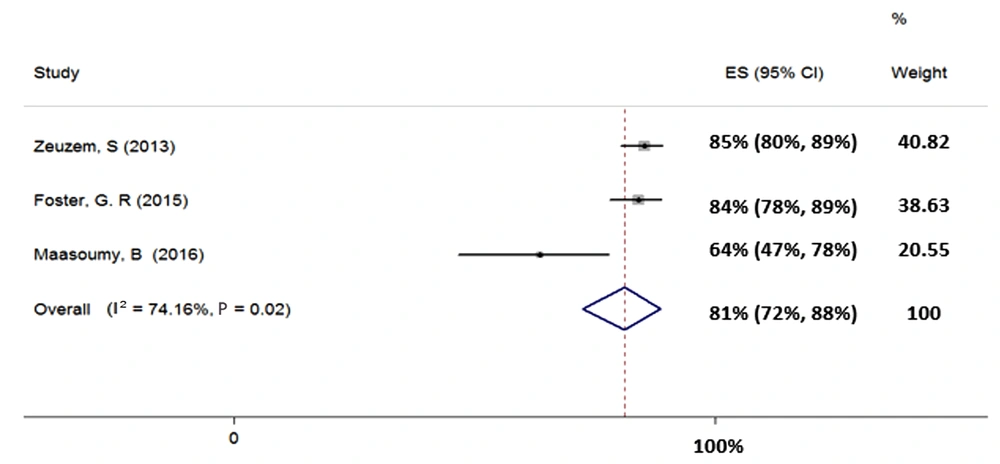
Three studies were included in the meta-analysis of the SOF + RBV regimen for 24 weeks in patients with HCV-3 (total sample size = 465). Heterogeneity was found in the results of the included studies (χ2 = 7.74, P = 0.02, I2 = 74.16%); therefore, the random-effects model was used. The SVR rate (95% CI) was calculated as 81% (72% - 88%) (Figure 5).
4.4.5. Treatment of HCV Genotype-3 with Sofosbuvir Plus Ribavirin and Pegylated-Interferon for 12 Weeks
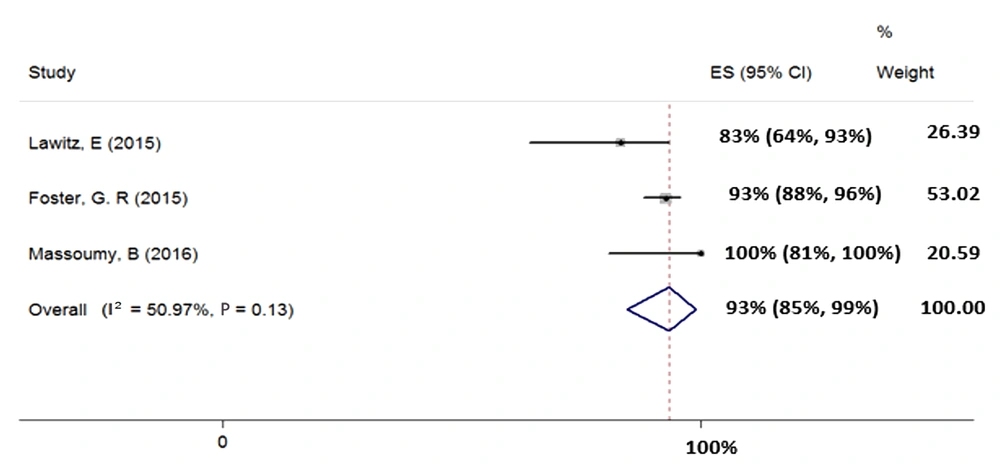
We included three studies (total sample size = 221) assessing the effect of 12-week treatment with SOF + RBV + PegIFN on patients with HCV-3. Heterogeneity was not found between their results (χ2 = 0.08, P = 0.13, I2 = 50.97%); therefore, we used fixed-effects model that pooled calculated the SVR rate (95% CI) as 93% (85% - 99%) (Figure 6).
5. Discussion
In this meta-analysis, we found that SOF + RBV ± PegIFN regimen could be effective in most patients with HCV-2 or 3 infections. Although the previous standard of care with PegIFN and RBV combination therapy was more effective in patients with HCV-2 or -3 infections than in those with HCV-1 or -4 infections (8, 9, 11) and resulted even up to a 90% SVR, the long-term antiviral therapy, the side-effects of PegIFN and RBV, and the contraindication of PegIFN and RBV in conditions such as cirrhosis made the treatment of patients infeasible with low treatment uptake and patient adherence (31). In recent years, the development of DAAs for the treatment of HCV infection has changed the standard of care for the treatment of patients with HCV infection (32-34). The introduction of SOF as a pangenotypic inhibitor of HCV NS5B polymerase was the first step in the improvement of HCV-2 or -3 treatment (32). The addition of SOF to RBV with or without PegIFN resulted in the increased response rate to therapy in patients with HCV-2 or -3, with a shorter duration of treatment and fewer treatment complications compared to PegIFN + RBV (19).
In this study, we pooled the data of study/study arms for two regimens of SOF + RBV and SOF + RBV + PegIFN, both for 12 weeks, in patients with HCV-2 infection. Interestingly, the response rate was not significantly different between the two above-mentioned regimens (92% vs. 95%), making the IFN-free SOF + RBV for 12 weeks superior for the treatment of patients with HCV-2 infection. Regarding the treatment of HCV-3 infection, three regimens of SOF + RBV + PegIFN for 12 weeks and SOF + RBV for 12 and 24 weeks were assessed. The treatment of HCV-3 patients with SOF + RBV for 12 weeks resulted in a suboptimal response rate of 55% while the prolongation of the combination therapy with SOF + RBV to 24 weeks increased the response rate to 81%. Furthermore, SOF + RBV + PegIFN for 12 weeks resulted in a 93% response rate in patients with HCV-3 and thus, this regimen was superior in terms of higher responses compared to the two other regimens of SOF + RBV in patients with HCV-3 infection.
The introduction and approval of daclatasvir (DCV), an NS5A inhibitor of HCV, were other steps in the optimization of the treatment of HCV-2 or -3 infection with an increase in the SVR rate to more than 95% (35). The regimen of SOF + DCV can be administered in non-cirrhotic and treatment-naïve patients with HCV-3 infection for 12 weeks while patients with a history of treatment or advanced liver disease should be treated with the addition of RBV and/or the prolongation of the treatment course. More recently, the combination of SOF/velpatasvir (VEL) was approved for the treatment of HCV infection as a pangenotypic regimen (32). Although these two IFN-free regimens are more efficient than the regimen of SOF + RBV ± PegIFN in patients with HCV-2 or -3, DCV and SOF/VEL are not available and affordable in most developing countries and resource-limited settings where the frequency of HCV infection is high. Fortunately, SOF, RBV, and PegIFN are manufactured in many countries as generic drugs at reasonable prices.
In conclusion, the combination of SOF and RBV with or without PegIFN for 12 weeks is highly efficacious (> 90%) for the treatment of patients with HCV-2 infection. However, for the treatment of patients with HCV-3 infection, only 12 weeks of SOF + RBV + PegIFN would result in > 90% treatment success. With all of these new regimens of anti-HCV treatment, there is great hope for the global elimination of hepatitis C by 2030.