1. Background
The TB mortality rate is estimated at about 37% according to the 2017 global tuberculosis (TB) report by the World Health Organization (WHO) (1, 2). Bacillus Calmette-Guérin (BCG) vaccine is the first vaccine against TB developed by two French scientists in 1921. The BCG vaccine contains weakened or attenuated forms of Mycobacterium bovis that naturally causes TB in animals such as cows. The BCG vaccine gives protection against severe forms of the infection, especially TB meningitis, but it is less effective against pulmonary TB (3).
Heparin-binding haemagglutinin (HBHA) is a surface adhesin that mediates the attachment of mycobacteria to lung epithelial cells and extrapulmonary dissemination of TB. The disruption of the hbha gene or coating surface of mycobacteria with anti-HBHA antibodies could hinder the dissemination and colonization of Mycobacterium tuberculosis (M. tuberculosis) in the spleen. Ag85a has a mycolyltransferase activity that participates in the biogenesis of trehalose dimycolate (cord factor) and cell wall integrity and is a part of secreted proteins in M. tuberculosis and the BCG culture filtrate. Due to the ability of this antigen in T cell proliferation and IFN-γ induction, it is considered a promising vaccine candidate (4, 5).
Cfp10 is a secretory protein and a virulence factor encoded by “region of difference 1” (RD1) of the M. tuberculosis genome that is absent in all strains of BCG. This region has a major role in the virulence properties of Mycobacterium bovis bacillus and seems to be an important target for protective CD4+ T cells immunity. Tb10.4 belongs to a low molecular weight protein of the ESAT6 family. The Tb10.4 gene is highly conserved in the clinical isolates of M. tuberculosis and it plays a significant role in mycobacterial pathogenesis. The expression of the Tb10.4 gene is downregulated in the attenuated strain of M. tuberculosis (H37Ra) compared to the wild strain (H37Rv). Previous studies on Tb10.4-based vaccines showed that Tb10.4 is an ideal vaccine candidate (1). Mtb32C is a C-terminal domain of mtb32A protein with strong activity in stimulating cytotoxic T cells (CTL). Many previous studies confirmed that these antigens show strong activity in stimulating immune responses, especially cell mediate immunity (the most effective response against TB), but, up to date, there is no study showing the effectiveness of these antigens together (6). Due to the global public health threat of TB and insufficient protection of BCG, developing a new and more effective vaccine candidate is desperately required.
Vaccine strategies that employ primary vaccinations of BCG with subsequent inoculation of DNA constructs encoding immunodominant antigens have been reviewed and all obtained results have shown the enhanced activity of T cell-mediated responses (7). Besides, previous studies have confirmed that Th1 cytokines like IFN-γ and IL-12 have a critical role in providing essential protection against M. tuberculosis infection. In contrast, Th2 cytokines like IL-4 and IL-10 have a suppressive effect. Surveying different modulating cytokines is an appropriate means to predict the potential stimulation of immune responses by a designated vaccine. In this study, three chimeric DNA vaccines containing the fusion genes (Mtb32C-HBHA, Ag85a-CFP10, and Ag85a-Tb10.4) were administered to BALB/c mice alone or in combination with BCG to evaluate immune-modulating cytokines including IL-4, IL-10, IL-12, TGF-β, and IFN-γ in order to investigate their effects as boosters in increasing the efficacy of the BCG vaccine as a prime vaccine (8, 9).
2. Methods
2.1. Mice
Thirty specific pathogen-free (SPF) female BALB/c mice (6 - 8 weeks’ age, 25 g weight) were obtained from the Razi Vaccine and Serum Research Institute (Mashhad, Iran). All mice were maintained in the SPF environment and all procedures performed were in accordance with the ethical policies of Mashhad University of Medical Sciences (ethics code IR.MUMS.REC.1393.160) and the guidelines of the Institutional Animal Care and Use Committees (10).
2.2. Construction of Chimeric DNA Vaccines
All recombinant vectors used were constructed in previous studies. In summary, the genome of Mycobacterium tuberculosis H37Rv strain was extracted and related genes including Mtb32C, hbha, Tb10.4, Cfp10, and Ag85a were isolated and cloned into the pCDNA3.1+ vector using polymerase chain reaction (PCR). Finally, three DNA vaccines (pCDNA+-Mtb32C-HBHA, pCDNA+-Ag85a-Cfp10, and pCDNA+-Ag85a-Tb10.4) were constructed and the purified vectors were injected into the tibialis anterior muscle of susceptible animals (11, 12). In our previous studies, each recombinant vector was given to BALB/c mice in a prime-boost manner but in this study, all of them were assembled and used as a single vaccine (9, 13-15).
2.3. Immunogenicity of Chimeric DNA Vaccines
Thirty female BALB/c mice were divided into three groups [empty vector (pCDNA3.1+) as the control group, chimeric DNA vaccines as the vaccine group, and BCG plus chimeric DNA vaccines as the BCG-vaccine group]. The BCG-vaccine group was first vaccinated subcutaneously with BCG (5 × 105 CFU in PBS) (Pasture institute, Iran) (16) and then after one month, they were inoculated intramuscularly with chimeric DNA vectors (100 µg in 300 µL of PBS) three times at two-week intervals (12, 17). The control group and the vaccine group were immunized intramuscularly with pCDNA3.1+ and three chimeric DNA vaccines, respectively, at a total concentration of 100 µg three times at two-week intervals (9, 12, 18).
2.4. Cytokine Analysis
Four weeks after the last immunization, eight mice from each group were sacrificed. First, the spinal cord was cut and then, the spleen was removed using sterile instruments. Their splenocytes were isolated, plated (3 × 105 cells per well), and cultured in 24-well plates containing RPMI-1640 medium enriched with 10% fetal calf serum and 1% penicillin and streptomycin (19). The cells were stimulated with the BCG vaccine (2 × 104 CFU) for 72 hours. After stimulation, the culture supernatants were gathered and interleukin-12 (IL-12), IL-4, IL-10, TGF-β, and IFN-γ were measured using ELISA kits (BD, Biosciences, San Jose, CA) according to the manufacturer’s instructions (19). The limits of detection of ELISA were as follows: 15 pg/mL for IFN-γ, 15 pg/mL for IL-12p70, 4 pg/mL for IL-4, 30 pg/mL for IL-10, and 8 pg/mL for TGF-β.
2.5. Statistical Analysis
The data were presented as means and standard deviations. Normality was evaluated using the Kolmogorov-Smirnov test. The differences between groups were assessed using the one-way ANOVA with P values of < 0.05 considered statistically significant.
3. Results
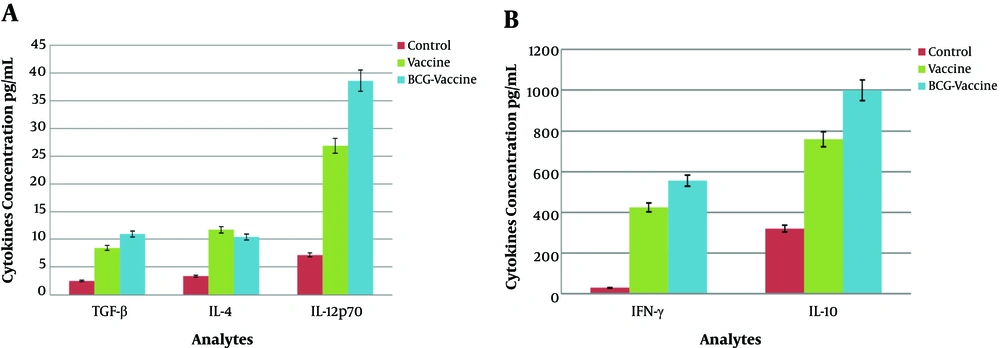
One month after the final immunization, the animals were killed. Cytokine productions were assessed in the stimulated culture supernatant using the ELISA method (BD Biosciences). The levels of different cytokines in each group are listed in Table 1. As shown in Figure 1 and Table 1, the levels of the measured cytokines were significantly higher in the vaccine group and BCG-vaccine group than in the control group (P < 0.05).
| Vaccination Groups | IL-12p70, pg/mL | IL-4, pg/mL | IL-10, pg/mL | IFN-γ, pg/mL | TGF-β, pg/mL |
|---|---|---|---|---|---|
| Control | 7.16±1.7 | 3.37±0.85 | 320.3±62.3 | 29.9±13 | 2.5±1.2 |
| Vaccine | 26.85±17.15 | 11.74±2.8 | 759.46±240.1 | 424.5±78 | 8.43±0.75 |
| BCG-vaccine | 38.56 ±16.05 | 10.42±1.8 | 999.74±277.2 | 555.5±43 | 10.96±2.8 |
Splenic cells from mice immunized with the BCG-vaccine had slightly higher levels of IL-4 (10.42 ± 1.8 pg/mL), TGF-β (10.96 ± 2.8 pg/mL), and IL-10(999.74 ± 277.2 pg/mL) secretion than those from mice only receiving DNA vaccines [IL-10 (759.46 ± 240.1 pg/mL), IL-4 (11.74 ± 2.8 pg/mL), and TGF-β (8.43 ± 0.75 pg/mL)], but differences were not significant (P > 0.05).
The levels of IFN-γ (555.5 ± 43 pg/mL) and IL-12 (38.56 ± 16.05 pg/mL) in the BCG-vaccine group were much higher than the levels in the vaccine group [IFN-γ (424.5 ± 78 pg/mL) and IL-12 (26.85 ± 17.15 pg/mL)] and the differences were significant (P < 0.001 and P < 0.05, respectively).
4. Discussion
Tuberculosis remains the main cause of death in many parts of the world. Reducing the prevalence rate of TB worldwide is the main gold of WHO; hence, much research is underway in this filed (20).
Several meta-analyses indicated that the efficacy of the BCG vaccine, as the only vaccine available against TB, is just 50% (21, 22). However, BCG is very effective against miliary disease and TB meningitis and confers partial resistance to leprosy and Buruli ulcer (23). The BCG efficacy is very limited in preventing adult pulmonary TB and cannot be used in immunosuppressed patients because of fatal disseminated infections that may occur. Keeping with these observations, the development of a more effective vaccine provides a useful solution to the TB threat (24).
In many endeavors, several vaccines, as primary vaccines to replace BCG or as boosters for BCG, have been evaluated, some entering clinical trials. Recent research has been promising for TB control in the future (25). Generally, a prime-boost strategy is a promising approach to induce protective cellular immunity, as its effectiveness has been previously confirmed in humans. Priming with BCG and boosting with a DNA vaccine encoding multiple antigens showed a broad stimulation in cytokines production and led to high levels of T-cell immunity. This strategy has entered clinical trials and it is an impressive way to stimulate long-time immune responses against M. tuberculosis (24). The most advantage of a DNA vaccine over other types of vaccines is related to its capacity in stimulating antigen-specific cytotoxic T lymphocyte (CTL) and long-term introduction of phase-dependent or poorly expressed antigens in BCG in order to improve the BCG vaccine (26).
T cell subsets play an important role in inhibiting TB infection and obtaining the optimal protection by inducing IFN-γ-secreting CD4+ Th1 cells. The best way of evaluating a vaccine’s efficacy is to identify immunological parameters. In this regard, the levels of several cytokines were assessed in the present study. IFN-γ and IL-12 play a central role in response to TB. Previous studies indicated that the disruption of IFN-γ and IL-12 genes caused extreme susceptibility to intracellular pathogens such as mycobacteria (27). Indeed, identifying the levels of IFN-γ and IL-12 is the most widely used method for the recognition of immune responses following vaccination. The balance between Th1 (IFN-γ, IL-12) and Th2 cytokines (IL-10, IL-4) is likely to influence the vaccine outcome (28).
IL-10 has an inhibitory effect on Th1 cells and blocking this cytokine facilitates the IL-12 production from monocytes by neutralizing antibodies and increasing the production of IFN-γ (29). Although IL-10 is a suppressive cytokine, it has been shown that own T cells (CD4+ effector T cells) (30) are an alternative source of IL-10 production; thus in our study, the high levels of IL-10 cytokine may be related to this issue (31, 32). Moreover, there is a body of evidence indicating that IL-10 has immunostimulatory effects on CD8+ Tcell cells. IL-10 in the presence of low doses of IL-2 promotes the proliferation of CD8+ T cells, which is believed to be involved in the successful elimination of mycobacterial infection and long-lasting control of the infection (33, 34).
TGF-β is an anti-inflammatory cytokine, which prevents the activation and proliferation of naïve TCD4+/TCD8+ cells, yet it has a contradictory effect on different cells. For instance, TCD4+ in the presence of TGF-β cannot differentiate, but TCD8+ cells following their activation produce TGF-β which induces its differentiation. In spite of the inhibitory effect of TGF-β on T cells, recent studies have shown that in certain circumstances, TGF-β facilitates the growth of Th17 cells as the main effector cell in secreting IFN-γ and activating macrophages (35, 36).
In the present study, mice were primarily vaccinated with BCG and then inoculated with three chimeric DNA vaccines, leading to high levels of IL-12 and IFN-γ production. These results demonstrate that the administration of such regimen leads to a significant increase in cell-mediated immune responses. In this work, the level of IL-4 as a Th2 biomarker was low, indicating that humoral immune responses were not effectively stimulated. The elevation of IL-12 along with TGF-β supports memory T cells generation and their maintenance. The administration of such DNA vaccines encoding Ag85a, HBHA, Mtb32C, cfp10, and Tb10.4 antigens can improve the efficacy of the BCG vaccine with a prime-boost strategy, as they are absent or poorly expressed in BCG (37, 38). Many studies have indicated that any mutation or disruption in the IFN- γ gene or its receptors increases susceptibility to intracellular pathogens such as mycobacteria. Cytokine analysis in our previous studies and this work indicated that the ratios of IFN-γ to Il-4 in Ag85a-Cfp10, Ag85a-Tb10.4, Mtb32C-HBHA, and co-administration with vaccines were 1.8, 27.31, 15, and 36.18, respectively. These results suggest that the combination of these three DNA vaccines is more potent than each one alone in stimulating immune responses (9, 13-15). In fact, these results were obtained in the absence of any adjuvants; therefore, continuing such experiments and analyzing different aspects of these new antigens will be hopeful.
This study showed that IFN-γ production by the three chimeric DNA vaccines was the most desirable outcome that eventually led to the induction of cellular responses. In addition, the induction of cytokines including IFN-γ, IL-12, and TGF-β was precisely correlated with enhanced BCG-mediated protective immunity. Consistent with previous studies, the ratio of IFN-γ to IL-4 in the BCG-vaccine group (BCG for priming and DNA vaccines for boosting) was higher than the ratio in the vaccine group, indicating that the cell-mediated immunity was more stimulated than humoral immunity. Our results showed that these chimeric DNA vaccines could enhance the effectiveness of the BCG vaccine and they could be used as boosters (39). In the present study, the co-administration of the three DNA vaccines encoding several immunodominant antigens were observed for investigating the efficacy of the currently used TB vaccine, BCG. However, the protective properties of these DNA constructs and their therapeutic effects remain to be examined in detail in future studies. Finally, the present results suggest that Mtb32C-HBHA, Ag85a-Cfp10, Ag85a-Tb10.4 fusion proteins could potentially be used as promising candidate antigens for future TB vaccine development.
4.1. Study Limitations
Because of limitations in the animal isolator, the animal challenge test was not performed; therefore, the levels of modulatory cytokines in the lung of mice were not measured. Humoral immunity is not effective against intracellular pathogens like M. tuberculosis; therefore, the levels of antibodies in the serum of immunized mice were not assessed. Furthermore, the generation of effector-memory T cell and the therapeutic properties of constructed vectors were not evaluated. Bioinformatics analysis of these new fusion antigens will be helpful in the identification of related B cell and T cell epitopes.
4.2. Conclusions
The most effective immune response against TB is cell-mediated immunity; hence, drawing attention to this point is very critical in vaccine development. Recent studies have indicated that the prime-boost strategy is a suitable way to access this goal. Based on the obtained results, the immunization of mice primed with BCG and then boosted with mycobacterial immunodominant antigens can induce effectively Th1 cytokines.