1. Background
Various Staphylococcus species are responsible for causing many diseases in humans (1). These bacteria are divided to two coagulase-positive and coagulase-negative and one of the most important groups of nococomial infections that are resistant to a wide range of beta-lactam antibiotics (2). Coagulase-negative staphylococcal (CoNS) isolates include S. epidermidis, S. hominis, and S. haemolyticus (3). Indeed, S. aureus is the main cause of soft tissue infections, septicemia, toxic shock syndrome, and deep postsurgical infections (4).
At the early stage, different genetic mutations in the S. aureus genome lead to the emergence of antibiotic-resistant strains. Variations in the nucleotides are responsible for the activity of some genes responsible for resistance and bacterial resistance to multiple antimicrobials, including beta-lactams (5, 6). A mutant variation of the gene named mecA, encoding for penicillin-binding protein 2a (PBP-2a) was responsible for the emergence of β-lactam resistance in mutant strains. A few antibiotics, such as mupirocin are still active against MRSA (7). Mupirocin has become known as the topical antimicrobial agent of drug choice for the elimination of S. aureus nasal carriage (5). In hospitals, at which MRSA is epidemic, the intranasal administration of mupirocin to both personnel and patients colonised with MRSA is considered to be suitable (8). In addition, mupirocin has been used effectively to prevent staphylococcal infections in haemodialysis and surgical patients (9).
Different phenotypic and genotypic methods are available for identifying different species of Staphylococcus aureus (10). In some studies, it has been reported that biochemical, immunological, and enzymatic methods, such as coglulase test and mannitol test, do not provide accurate information for the identification of different strains of staphylococcus (11). Different antibiotic disks (such as novobiocin and nalidixic acid) and biochemical methods (such as PYR test, ornithine decarboxylase test, acid production from maltose, trehalose, mannitol, and sucrose) are used to identify and isolate different species of CoNo (9). Thus, to overcome these drawbacks, PCR-based methods have been developed for rapid and sensitive detection of the pathogens (12). The annealing and melting properties of DNA have paved the way for progression of many molecular deteciton methods (11, 13). The melting curve pattern of the DNA samples was used for this purpose. The test was first described by Carl Wittwers who defined a molecular diagnosis based on the relationship between temperature and the level of DNA denaturation termed as melting curve (14). The melting curve characterized by rising temperatures and changes in the slope of the curve indicates the temperature performance in relation to the melting point. The melting point is the time durin which at least 50% of the DNA has been denatured (15, 16). The separation of the two strands of DNA by heating (melting) is identified by a fluorescence dye, such as SYBR Green that is bound specifically to the double-stranded DNA (14).
2. Objectives
The main objective of the current study was to configure MCA, based on real time PCR to design the specific primers of Staphylococcus species. Also, by determining the analytical sensitivity and specificity, different strains of resistance to mupirocin and methicillin could be detected in S. aureus.
3. Methods
3.1. Staphylococcus Species
In this experimental study, which was done on clinical and standard isolates of Staphylococcus bacteria that were stored in the microbiology laboratory of the University of Medical Sciences of Hamadan in 2017, S. aureus, S. saprophyticus, S. epidermidis, and S. hemolyticus were investigated by conventional methods consisting of colony morphology, catalase, Gram stain morphology, and coagulase testing. Phenotype analysis of methicillin and mupirocin resistance were performed, respectively, using cefoxitin (30 µg) and mupirocin disk (200 µg) (purchased from the Mast England co.), according to the CLSI guidelines (17).
3.2. Preparation of DNA Extraction
In order to extract genomic DNA, the PR881614 kit (CinnaGen, Iran) was used, for which crude DNA was prepared in accordance with the manufacturer’s instructions. At first, a single colony was transferred in 5 mL BHI broth and incubated for 24 hours at 37°C. Next, 1.5 mL of the suspension was transferred in a sterile 1.5 mL Eppendorf tube and then centrifuged for 10 minutes (4500 rpm). DNA was extracted accompanied with lysozyme and the sediment from the centrifuged bacteria in BHI. DNA concentration and light absorption were measured in the wavelength of 260 and 280 NM by a spectrophotometer Nanodrop (A & E lab, U.K) (18).
3.3. Design of Prime
Genetic targets for primer design included: The 16S rDNA gene of all members of the genus Staphylococcus, the ITS of S. aureus, the sap of S. saprophyticus, the PhoP of S. epidermidis, the mvaA of S. hemolyticus, and also two resistance genes (including the mecA of Staphylococcus genes resistance to methicillin and the MupA of mupirocin resistance). After selecting the targets for primer design, databases of each bacteria was searched from the NCBI, DDBJ and EMBL database. Next, in order to align information of each gene, MEGA version 4 software (MEGA, Inc., USA) was used. To evaluate the annealing temperature and designed Tm primers, Oligo version 7 (Molecular Biology Insights, Inc., USA) and Gene Runner version 6 (Gene Runner, Inc., USA) software were used. By using NCBI databases, Blast was performed with human, fungus, virus samples, and other microorganisms to make sure about the specificity of the primers' performance (Table 1).
| Bacteria | Sequence of Primers | Gene | Tm.P | Product Size, bp | References |
|---|---|---|---|---|---|
| Universal | F: ACTTCGGGAAACCGGAGC | 16 S rRNA | 79/57 | 190 | This study |
| R: ACCGTGTCTCAGTTCCAG | |||||
| S. saprophyticus | F: ACGGGCGTCTCGATAGAAA | sap | 76/49 | 136 | This study |
| R: AACGATTCTGGTTAAGTTGCC | |||||
| S. epidermidis | F: ATTGTAATTTCATTTGTCGCAC | phop | 74/2 | 98 | This study |
| R: AGTACTCGTGAACTCATCGC | |||||
| S. haemolyticus | F: GGTCGCTTAGTCGGAACAAT | mvaA | 78/2 | 271 | This study |
| R:CACGAGCAATCTCATCACCT | |||||
| Methicillin resistance | F: GGCTCAGGTACTGCTATCCAC | mecA | 76/6 | 297 | This study |
| R:AACGTTGTAACCACCCCAAGA | |||||
| S. aureus | F: GTTAGAGCGCACGCCTGATA | ITS | 83/73 | 155 | This study |
| R: AATGGTGGAGACTAGCGGGA | |||||
| Mupirocin resistance | F: GCGACGGTTTAGTTAATGCA | mupA | 47/83 | 76 | (19) |
| R: TGAACAATACCAGTTCCTTCTGA |
3.4. PCR Amplification
The reaction solution was prepared in a volume of 20 µL, which included 10 µL of master mix (Ampliqon Germany), 1 µL forward primers and 1 µL reverse primers (10 pmol), 1 µL of sample DNA and sterile DW. DNA amplification was carried out by Eppendorf, Germany PCR system thermocycler with thermal cycling conditions consisting of an initial denaturation step at 94°C for five minutes, followed by 30 amplification cycles including denaturation at 94°C for one minute, annealing at 58°C for one minute, and extension at 72°C for 10 minutes. Positive and negative controls included reference strain of Staphylococcus, and clinical and standard strains obtained from clinics. The true-negative controls included Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC27853.
3.5. Evaluation of Sensitivity and Specificity of the Primers
To examine the sensitivity of the designed primers, a bacterial standard with concentration of 0.5 McFarland (1.5 × 108 cfu/mL) was provided in serial dilutions of 108 to 101 cfu/mL in triplicates during three consecutive days. Real-time PCR test was performed for all the dilutions. In order to test the specificity of the primers, positive control strains, including Staphylococcus and human DNA, and negative controls, including the DNA of other bacteria, such as strains A. baumannii ATCC 19606, strain E. coli ATCC 25922, and strain P. aeruginosa ATCC 27853, were used as a negative control. Melt curve profiles were assessed and analyzed using the ABI step one software version 2.3 (ABI Thermo Fisher Scientific, Inc., USA).
3.6. Real Time PCR Method
The amplification of the genes was carried out using the real time PCR (ABI step one plus, USA). Reactions were carried out in a total volume of 20 µL including Master Mix PCR SYBR Green 10 µL, 1 µL of each primer (10 pmol), 1 µL of bacterial DNA, and distilled water (DNAse and RNAse free). The cycling conditions were as follows: Denaturation at 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C. In order to draw a melting curve for the separation of the genes, the temperature cycle was as follows: 95°C for 15 seconds, 58°C for one minute and 95°C for 15 seconds. Finally, melt curve profiles were assessed and analyzed using the ABI Step one software.
3.7. Sequencing
The PCR products were purified and sequence for identification of Staphylococcus spp. was determined by the Korea Bioneer Company (Representative Pishgam Company/Iran).
4. Results
4.1. Species and Antibiotic Gene Resistance Identification by PCR
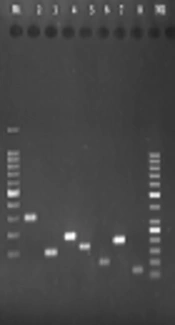
The its gene primers with length of 155 bp, phop gene primers and length of 98 bp, sap gene primers with length of 136 bp, mvaA gene primers with length of 271 bp, mecA gene primers with length of 297 bp, mupA gene primers with length of 76 bp, 16srRNA gene primers with length of 190 bp, were successfully amplified for identification of all the staphylococcal species chosen for this study. Gel electrophoresis confirmed that the amplicons were of expected sizes (Figure 1).
Gel electrophoresis of PCR products. Lane 2: mecA gene with length of 297 bp, lane 3: sap gene primers with length of 136 bp, lane 4: 16srRNA gene with length of 190 bp, lane 5: its gene with length of 155 bp, lane 6: phop gene with length of 98 bp, lane 7: mvaA gene primers with length of 271 bp, lane 8: mupA gene with length of 76 bp, lane M1: DNA size marker (100 bp), lane M2: DNA size marker (50 bp).
4.2. Analytical Sensitivity of Primers
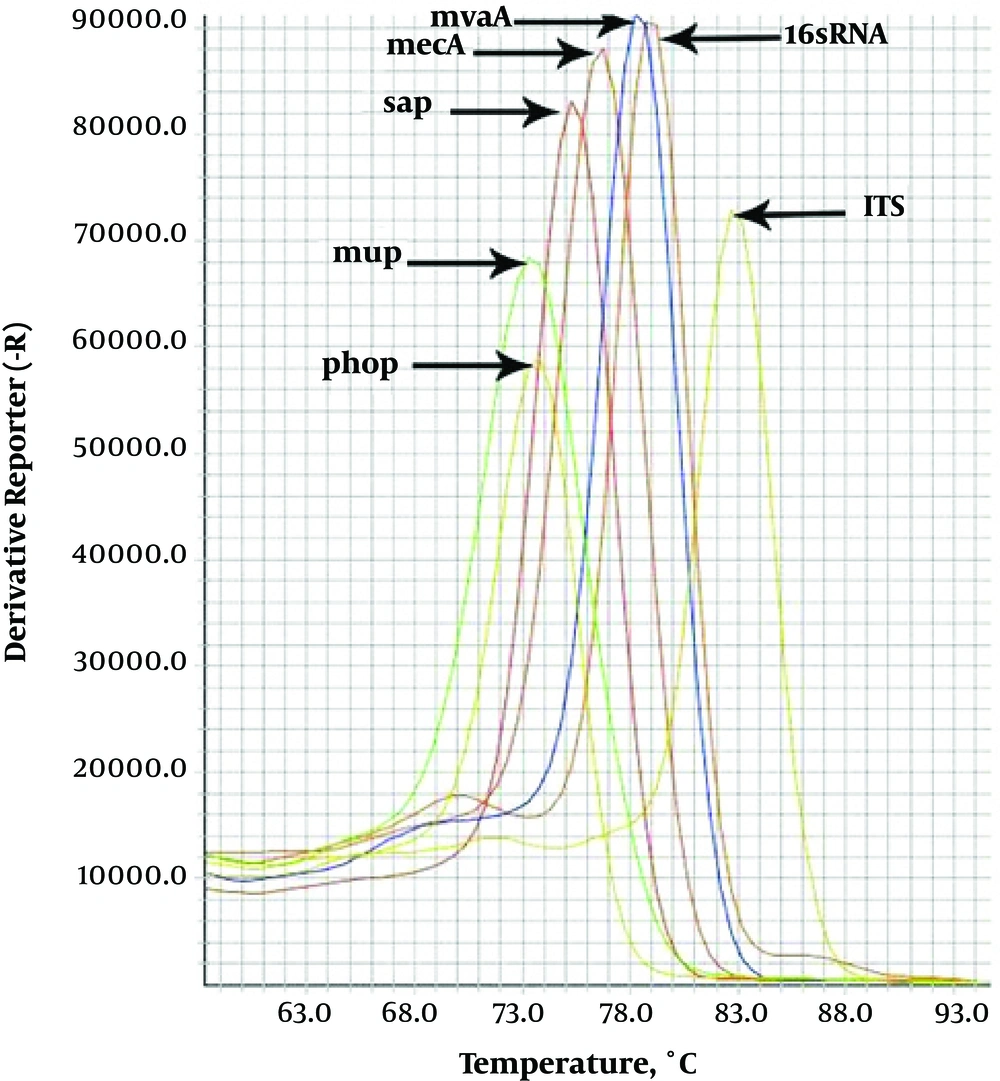
In all dilutions, the observed melting temperatures shown in the melting curves of gene amplification were equal to 83.79°C for ITS-gene, 74.2°C for phop gene, 76.49°C for sap-gene, 78.2°C for mvaA gene, 79.57°C for 16srRNA-gene, 74.83°C for mupA gene and 76.6°C for mecA gene (Figure 2); all of these melting temperatures were completely consistent with the results of primer blasts in the NCBI database. The analysis of the threshold melting curves of the intended genes indicated a successful onset of gene replication in all prepared dilutions in different cycles. Obtaining the standard concentrations in the dilutions with ratios of 108 to 101, it was found that the simulated primer has the ability to identify less than150 CFU bacteria for S. aureus, S. epidermidis, S. saprophyticus and 16srRNA gene. Fifteen CFU bacteria for S. haemolyticus, less than 1500 CFU bacteria for mecA and mupA resistant gene.
Real-time PCR assay simultaneously detects seven different genes in staphylococcus. Data from individual reaction mixtures, each containing DNA from S. aureus, S. epidermidis, S. haemolyticus, S. saprophyticus, methicillin resistant Staphylococcus and mupirocin-resistant staphylococcus strains, are presented in a single graph to show the separation between individual amplicon melting curves.
4.3. Analytical Specificity of Primers
The temperatures observed at the end of the reaction according to the Melting curves showed that the primers used in the present study were not able to identify other bacteria, such as, Streptococcus pyogenes, Enterococcus fecalis, and Acinetobacter baumannii.
4.4. Sequencing Analysis
The results of the blast of the intended genes products in staphylococcus bacteria indicated that the simulated primers were non-homologous with other bacteria. Furthermore, the results of the alignment exhibited a very appropriate sensitivity and specificity for identification of the intended bacteria.
5. Discussion
Staphylococcus aureus and CoNS are considered as the most important causes of nosocomial and community-acquired infections. Additionally, they present the most frequently isolated microorganism in CoNos contamination (3).
In this study, isolation of Staphylococcus aureus and CoNo on standard strains based on the MCA method and the DNA melting temperature of the genes studied (16srRNA for Staphylococcus aureus, nuc for Staphylococcus aureus, mupA for resistance to mupirocin and mecA for resistance to methicillin) was done (19). However, the potency of the simulated primers was determined with respect to the different DNA dilutions, optical absorption ratios, and CTs observed in amplification curves. Considering these values, the specificity and low error rate of this method will be more evident than other conventional phenotypic methods, such as disc diffusion. In the study of Skow et al. (20) various Staphylococcus species were identified by the MCA assay based on real time PCR. Their results showed that by using precise molecular methods, such as real time PCR, it is possible to detect CoNS species from S. aureus. Also, in another study, Shariati et al. (21) compared disc diffusion and real time PCR methods to differentiate between methicillin-resistant and methicillin-sensitive S. aureus in blood samples.
The information on dilutions prepared in the current study along with its significance is available in Xing et al.’s research (22), which studied a variety of bacteria, including S. aureus. In the present study, DNA melting method was used to identify different species and genus; accordingly, the most sensitive dilution was 101, which is not an appropriate index for identifying S. aureus. However, this index was higher in other bacteria, according to previous studies, which was inconsistent with the present study.
Besides, there was a logical correlation between the concentrations obtained for DNA diluted by 0.5 McFarland, and the onset of cycles was obvious. This consistency can be appropriate for selecting the target site. In DNA melting based on real time PCR, one of the most important issues that should be considered in designing a method that could be used to select an appropriate and sensitive target site for the simulation of the primer. Forghani et al. (23) showed that selecting an appropriate target site in the DNA melting method can help identify bacteria in the dilution ratio of 101. Selection of appropriate dilutions and accuracy of the process are indicators of the similarity of both studies. Clifford et al. (24) intended to determine methicillin-resistant Staphylococcus strain; they found that the simulated specific primers could identify bacteria in the dilution ratio of 102, which was not consistent with the results of the study. These studies not only obtained the best melting temperature yet also analyzed the specificity of the simulated primers in addition to their sensitivity.
The sensitivity and specificity of molecular methods can be analyzed by standardizing the sequencing process. One of the characteristics of DNA melting techniques in Real-Time PCR is their ability of typing and categorizing different strains. Krawczyk et al. (25) used melting profile simulation along with PFGE method to increase its sensitivity. In the present study, the simulated primers were not able to identify similar strains and species after determining their melting temperatures, which was lower than the observed temperatures; this can express the analytical specificity of primers with respect to their analytical sensitivity.
The presence of expansion of methicillin resistant Staphylococcus aureus strains (MRSA) exhibit the importance of specific molecular methods. Patients infected with MRSA have the highest risk for expanding Staphylococcus aureus infection in the society (26). The most common method for detecting these bacteria are biochemical and phenotypic identification tests using culture media and commercial kits. These methods are problematic in terms of time, complexity, and accuracy (19). Therefore, to overcome this problem, PCR-based methods are used for immediate and accurate detection of these bacteria. Real-time PCR method can identify all MRSA strains within less than three hours, which is completely consistent with the method used in the present study.
One of the limitations in the MCA method was the inability to simultaneously detect coagulase negative and coagulase-positive staphylococci in a common infection. Furthermore, this method is configured based on the melting temperature of the DNAs and should utilize appropriate concentrations and primers and DNA samples to act as inhibitors. Another limitation of the present study was the lack of use of clinical specimens, as performing MCA on a standard strain with clinical specimens and mixing different bacteria is quite different. Once the sensitivity and specificity of the primers used is known, they are able to detect susceptible bacteria resistant to a mixture of different bacteria.
5.1. Conclusions
The current research effectively developed and designed a melting curve analysis (MCA) based on multiplex by real-time PCR assay for rapid testing of Staphylococci, and concurrently detected mupirocin resistance and methicillin resistance. This analysis offers clinical laboratories a new, rapid, simple, sufficient, specific, and precise means. Its application will permit the rapid determination of methicillin resistance and mupirocin resistance in settings where it is utilization and confidently will prevent the extensive dissemination of methicillin resistance and mupirocin resistance through first and consistent detection. Finally, it is suggested that the real time-PCR dependent methods have more sensitivity and specificity compared with the DNA melting curve analysis method, and by introducing more clinical isolates, the predictive value of each method was also investigated.