1. Background
Cervical cancer is the second most common cancer and cause of mortality in women worldwide. Various environmental and genetic factors are involved in the etiology and pathogenesis of the cervix, which leads to the transformation of epithelial cells. Human papillomavirus (HPV) “a DNA virus from the Papillomaviridae family” is the most important and known environmental factor for causing cancer and is in fact regarded as an essential factor for this type of cancer (1).
According to studies, other environmental factors for causing cancer include having the first sexual intercourse at young ages, having frequent intercourses, having multiple sexual partners, taking oral contraceptive pills and smoking. About 80 subcategories have so far been identified for this virus, out of which 20 are involved in causing cervical cancer, including subcategories 16, 18, and 25 (2).
Until now, over 100 different types of HPV have been identified using molecular methods. About 30% to 40% of the various types of this virus cause mucosal contamination, particularly in the anogenital tract. HPV is classified into three types in terms of their potential to cause cancer: high-risk, low-risk and intermediate-risk. Most cervical-contaminating HPV in the anogenital tract belong to the high-risk type and are able to develop cervical cancer (cervical carcinoma). From the virological point of view, HPV belongs to naked virus types (non-enveloped) with a 20-facet capsid, which has 8 basic and regulatory genes together with 2 structural genes of L1 and L2 in capsid with delayed expression (3).
The capsid protein gene is common in almost all different types of HPV and is considered as the basis for isolating the virus from the patients’ samples in most molecular methods. Of the 8 regulatory genes, e6 and w7 genes determine the virus’s ability to transform a lesion into cervical carcinoma. Thus, the first step to identify the presence of the virus in the samples from the cervix or other samples collected from patients is to extract the DNA of the virus. Considering the fact that infection with viruses is the leading cause for about one fifth of human cancers, HPV is known as one of the main oncoviruses which causes lesions not only in the anogenital areas, but also in the respiratory tract and lungs, mucosa and skin of head to neck, while types 16 and 18 of this virus cause cancer (4, 5).
Anogenital infections appear in the form of warts, condyloma and cervical intraepithelial neoplasia (CIN) (6-8).
2. Objectives
Since no reliable culturing method or serological technique is available for detecting HPV infections, and as the morphological studies alone are not highly sensitive to detect the infection even by employing state-of-the-art techniques such as liquid-based technique, applying molecular methods such as PCR on infected tissues would be very helpful. That is why these methods have been highly focused on in the present study.
3. Methods
3.1. Sampling
A total of 97 paraffin samples were collected from cancerous and precancerous genital lesions (after macroscopic examination and pathologist confirmation) from men and women (range: 20 - 60 years, mean age: 35 ± 8.1 years) in four hospitals’ pathology ward in Gorgan, North-East of Iran, during November 2017 - June 2018, after recording demographic information. Six benign lesions of the genital area were considered as negative control. Of the blocks, a fraction of 4 µm was prepared and was fixed on the microscope slide. These fractions were stained using hematoxylin and eosin method and were then approved by a pathologist. Afterward, the viral DNA was extracted using commercial nucleic acid extraction kits (Rosch, Italy) in accordance with the manufacturer’s protocol. The extracted samples were finally investigated in the wavelengths of 260 and 280 nm and their purity was assessed based on A260/A280 ratio.
3.2. Determining HPV Genotype
3.2.1. Designing Primer for Proliferation by Polymerase Chain Reaction
After designing a pair of primers (Table 1) for determining the presence of HPV with the help of software (CLC Main, CLC Main USA, Workbench 6) and investigating them thermodynamically, the AB-Anahitica kit (Germany) was used for further investigations.
| Primer Sequence | Primer | Size of the Fragment |
|---|---|---|
| 5’-ACACAACTGTGTTC-3’ | Forward primer | 139-145 bp |
| 5’-CAACTTCATCCACGTTCACC-3’ | Reverse primer |
3.2.2. Polymerase Chain Reaction (PCR)
The mixture from PCR reaction was prepared to the amount of 25 µL, consisting of 20 µL of master mix and 5µL of the DNA of the pattern, in order to undergo another reaction. The thermal cycles of PCR reaction are provided in Table 2.
| Number of Cycles | Temperature, °C | Denaturation |
|---|---|---|
| 1 | 50 | 2 minutes |
| 95 | 10 minutes | |
| 50 | 95 | 30 seconds |
| 50 | 30 seconds | |
| 72 | 30 seconds | |
| 1 | 72 | 5 minutes |
3.2.3. Post PCR
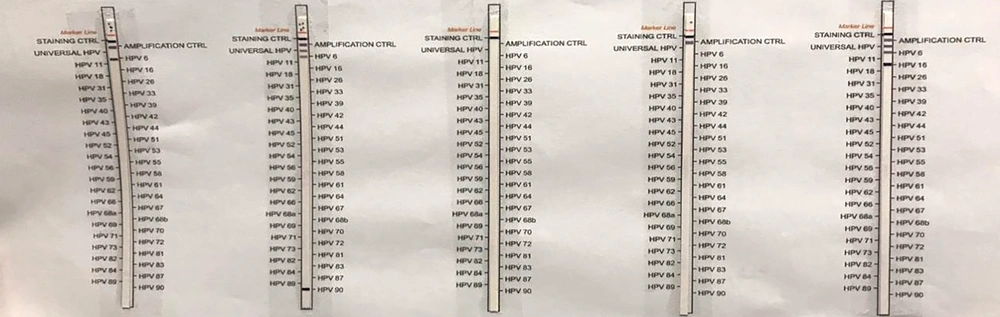
Once the PCR cycle was completed, 10 µL of the PCR product was mixed with 20 µL of the denatured solution and the product was incubated in the Tray for 5 minutes under room temperature. Then, 2 mL of the HyB-1 solution of 42°C were added to the mixture in the tray. After that, a special stripe for HPV genotyping was placed in the same tray, and the tray was shaken for 1 hour in the water bath.
Next, the tray was completely emptied and was again shaken in the water bath. The diluted solution was then added to it. The tray was emptied once more upon completion of this stage. In the next step, 2 mL of the rinse solution were added to the stripe within the tray, and the tray was placed on the rotator for 2 minutes after washing the stripe. This step was repeated twice. Finally, 2 mL of the staining solution were added to the tray and the product was incubated in dark for 5 - 10 minutes until the bonds appeared on the stripe. In the end, the Stop solution was used to terminate the reaction on the stripe.
4. Results
4.1. Results of Determining HPV Genotype
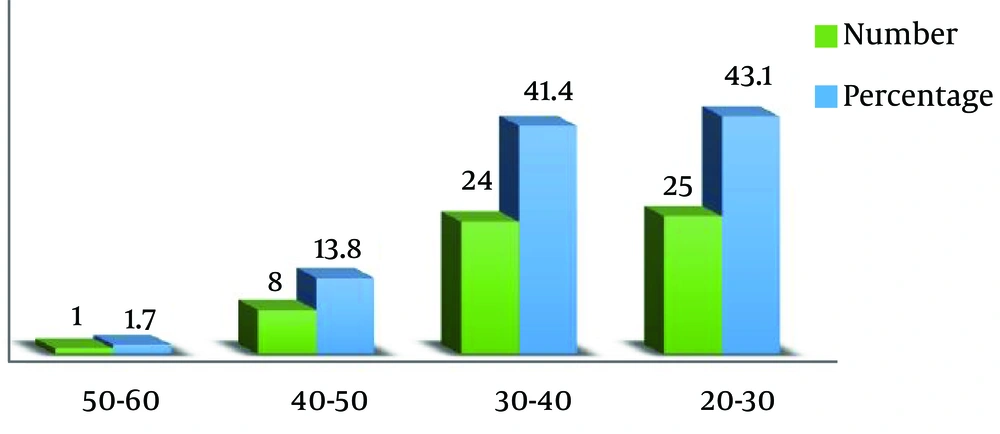
Of the 97 samples which were totally studied, 58 samples (59.8%) were reported to be positive and 39 samples (40.2%) were reported to be negative (Figure 1). The highest frequency of HPV in terms of gender was observed in women (89.6%), which shows that women are infected by the HPV virus much more than men. The highest frequency of HPV in terms of age was observed in the age range of 20 - 30 years (43.1%), which shows that infection by HPV decreases upon the increase in the age and that the risk of infection is higher among the youth (Figure 2).
4.2. Results of Categorizing Various Types of HPV
4.2.1. Categorizing Various Types of HPV Based on Who System
According to WHO categorization system, various types of HPV are classified into three categories of high-risk, low-risk and high-risk strain based on their genotypes and their pathogenicity degree and severity. Of the 58 samples which were reported as positive, 33 samples (56.9%) were infected by high-risk HPV types and 25 samples (43.1%) were infected by low-risk HPV types. Moreover, of this amount, the highest frequency was observed in women (60.4%) and belonged to the high-risk category, meaning that most patients were under the high-risk category (Table 3).
| Type | Women | Men | Total |
|---|---|---|---|
| High-risk | 32 (60.4) | 1 (20) | 33 (56.9) |
| Low-risk | 21 (39.6) | 4 (80) | 25 (43.1) |
aValues are expressed as No. (%).
4.2.2. Categorizing Various Types of HPV Based on IARC System
Of the total 97 patients, 6 genotypes could not be classified according to IARC categorization. Therefore, these six patients were excluded from categorization. Furthermore, 39 samples were reported to be negative in terms of containing HPV (Table 4). The highest frequency for the genotype of the cancerous HPV was classified under Group 1 based on the IARC categorization system.
| Type | Women (Negative Samples = 37) | Men (Negative Samples = 2) | Total (Negative Samples = 39) |
|---|---|---|---|
| Group 1 | 29 (63) | 1 (16.7) | 30 (57.7) |
| Group 2A | 4 (8.7) | 0 | 1 (1.9) |
| Group 2B | 1 (2.2) | 0 | 4 (7.7) |
| Group 3 | 12 (26.1) | 5 (83.3) | 17 (32.7) |
aValues are expressed as No. (%).
4.2.3. The Frequency of Various Types of HPV
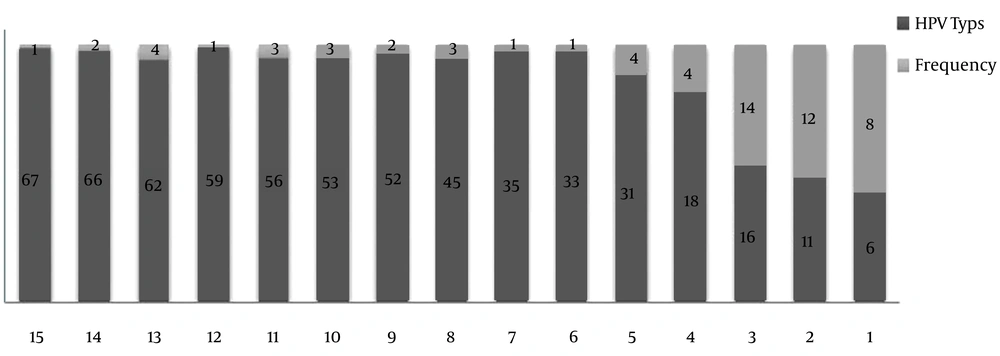
As per various types of HPV, the highest frequency (14 cases) was observed in type 16 (22.2%), and the lowest frequency was observed in types 33, 35, 59, and 67 (1.6%) (Figure 3).
5. Discussion
In the present study, 59.8% of samples were reported as positive in terms of containing HPV, which shows a high amount of infection by this virus.
In two studies (9-11), the DNA of HPV was found in 75% of the patients. Likewise, researchers in 2012 and 2005 reported that the rate of infection by HPV in patients with bladder cancer was 35.6% and 34.7%, respectively (12, 13), which has a lower frequency in comparison with the findings of the present study.
Comparing the results of the present study with those of other studies carried out in other countries, it could be realized that the rate of infection by HPV in Iran is higher than countries such as the Netherlands (15.2%), Greece (12%), Turkey (4.8%) and Germany (19%) (12). It seems that the risk of infection by genital HPV in these countries is lower due to timely visits to health centers in case of possible problems in addition to precise prevention and control programs used in these countries to reduce the risk of chronic sexually-transmitted infections.
In the present study, the highest frequency of HPV in terms of age was observed in women, which indicates higher infection by HPV in women compared to men. Age has always been a major factor in infection by HPV; however, in some countries, it seems that infection decreases with the increase in age. Some studies have revealed that women younger than 25 are more likely to be infected by this virus (14), which is consistent with the findings of the present study.
In this study, the highest frequency of HPV in terms of age was observed in the age range of 20 - 30 years (43.1%), which shows that infection by HPV decreases as the age increases, therefore the risk of infection is higher among the youth.
In a study conducted by Hashemi Dezfoli and Ghane (15), no significant relation was observed between the frequency of HPV and the age of the patients, which is in contradiction with the findings of the present study.
In this study, the highest frequency among the various types of HPV was found for type 16, which is consistent with the results of other studies carried out worldwide and in Iran (16).
Additionally, this type is the most common type of HPV reported among the cancerous samples collected from patients in Mazandaran and Yazd provinces in Iran (17).
HPV types 16 and 18 are the most common types involved in causing cervical cancer and are therefore known as high-risk types. Type 16, however, ranks first among factors leading to cervical cancer. Type 16 is observed in 60% of all cervical cancers, while type 18 is found in about 10% to 20% of the cases (18). The significance of type 16 has also been studied in causing other cancers such as breast cancer, lung cancer and prostate cancer (19-21). Types 6 and 11 are regarded as low-risk types which are the risk factor for genital warts.
Moradi et al. conducted a study on 308 swab and Pap smear samples from women in the Northern province in Iran and found out that 76 samples are positive in terms of containing HPV genome (22). Of these samples, 22 cases (5.8%) were HPV-16 positive, 15 cases (4%) were HPV-18 positive and 33 cases (10.3%) contained genotypes other than HPV-16-18. This is while in the present study, HPV-16 was reported to be positive in 22.2% of cases and HPV-18 in 6.3% of cases, which is much more than what was found by Moradi et al. (22) in this regard.
Jabarpour et al. in 2008 reported that the prevalence of type 31 among the HPV-infected samples was more than that of type 18 in Iran (23), while in the present study, no difference was spotted between types 31 and 18 in terms of their frequencies (6.3%). These findings can support the hypothesis that the frequency of circulating types of a population can be different from that of other communities.
Hassani et al. of Iran, have reported the highest amount of viruses in the cervical mucus to belong to the high-risk category (24), which is consistent with the findings of the present study.
Over recent years, scientific centers around the world have paid more attention to producing anti-HPV vaccines (25). Due to the relatively high prevalence of this virus in the cancer tissues in our country, it is recommended to use anti-HPV vaccines in the high-risk population. In addition, certain types of interferons are used in treating HPV-related lesions, but the prerequisite to using this treatment is to detect the presence of infection in the affected tissues. Hence, performing molecular tests together with other studies seems to be essential on precancerous and cancerous lesions.
5.1. Conclusions
This study proved that PCR and pro PCR are proper, accurate and sensitive methods with specificity to detect HPV. Besides, high-risk types of this virus are the main cause for contamination of cervical mucus in the anogenital area in most cases, particularly in case of precancerous and cancerous lesions. Like many similar studies, the findings of the present study confirm that HPV-16 is the most common virus type involved in infecting cervical epithelia.