1. Background
It is hypothesized that nanoparticles in calcified specimens may be a kind of bacteria called “Nanobacteria”. Some calcified structures in the human body can cause various diseases including gallstones and gallbladder inflammation (1), kidney and urinary stone formation (2-5), Alzheimer’s disease (6), vascular plaque, arterial heart disease (7), polycystic kidney disease (PKD) (8), dental calculus (9), prostatitis (10), psammoma bodies in ovarian cancer (11), calciphylaxis (12), and other diseases (13, 14). These nanobacteria can cause such important diseases due to the unbelievably sturdy apatite shells of these organisms. In many studies, researchers claimed that nanobacteria could accumulate different minerals as nidi under in vivo and in vitro conditions. Nanobacteria by these processes can create a protecting layer on the cell membrane that inhibits the penetration of any antinanobacterial agent into its structure. The lack of developing methods for growth inhibition has led these novel nanoscale bacteria to cause many problems such as increased resistance to antibacterial agents and increased associated diseases.
Calcification is one of the main causes of atherosclerotic plaque formation (15). It has been suggested that regulatory mechanisms such as osteogenesis (16, 17), stimulation of vesicle-calcification by lectins (18), and mast cell and macrophage-induced calcification (19) are involved in vascular calcification. Some studies have shown that high-molecular-weight calcified particles can act as nidi and accumulate minerals such as calcium to ultimately cause the calcification of soft tissue and develop diseases such as atherosclerosis (20, 21). Concerning the pathologic factors involved in atherosclerosis, it has been suggested that nanobacteria are possibly the candidate infectious agents that could form a hard apatite shell at physiological levels to cause plaque formation in vessels.
2. Objectives
The main purpose of this study was to isolate and identify nanobacteria in patients with atherosclerosis.
3. Methods
We collected 14 vascular plaques from patients with atherosclerosis who had undergone surgery at Sina Hospital, Tehran, Iran, from February 2016 to June 2016. For the isolation of nanobacteria-like structures (NLSs), the homogenized vascular tissue samples were filtered through 0.22-μm membrane filters. Then, Dulbecco’s modified eagle medium (DMEM; Gibco) was added to the precipitates obtained from centrifugation (at 20000 g for 40 minutes). The suspension was cultured in six-well plates with each well containing 5 mL of DMEM supplemented with 10% fetal bovine serum (FBS). Culturing was carried out using humidified air at 37°C with 5% CO2 and 95% air (LAB-LINE BI65, USA). A medium containing 5 mL of DMEM and 10% FBS (FBS) but without vascular plaques was considered as the negative control. Calcifying nanoscale particles (CNPs) growth was assessed by turbidity analysis at the 650-nm wavelength. Based on optical observation obtained from CNPs growth in 60 days, the positive plates were selected and samples containing white-colored sediments in the bottom of the wells were used for electron microscopic analyses including SEM (LEO 1450 VP, Germany) and TEM (LEO 912 AB, Germany). In the SEM study, floating and adherent materials in a culture plate were examined separately. Before microscopic examinations, the samples were prepared by methods suitable for biological samples. Then, the samples were analyzed by XRD and SEM/EDAX.
4. Results
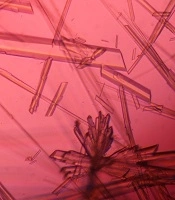
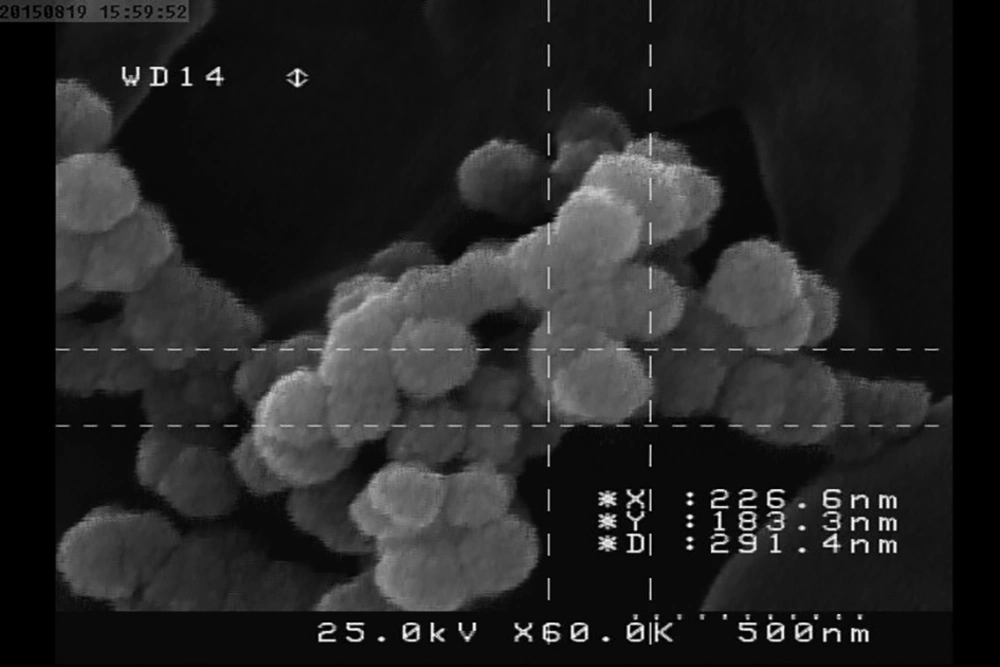
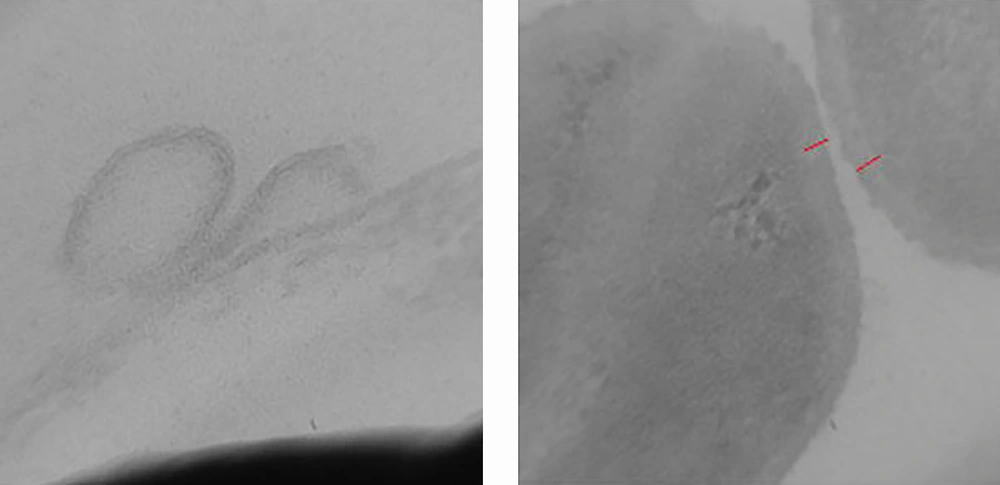
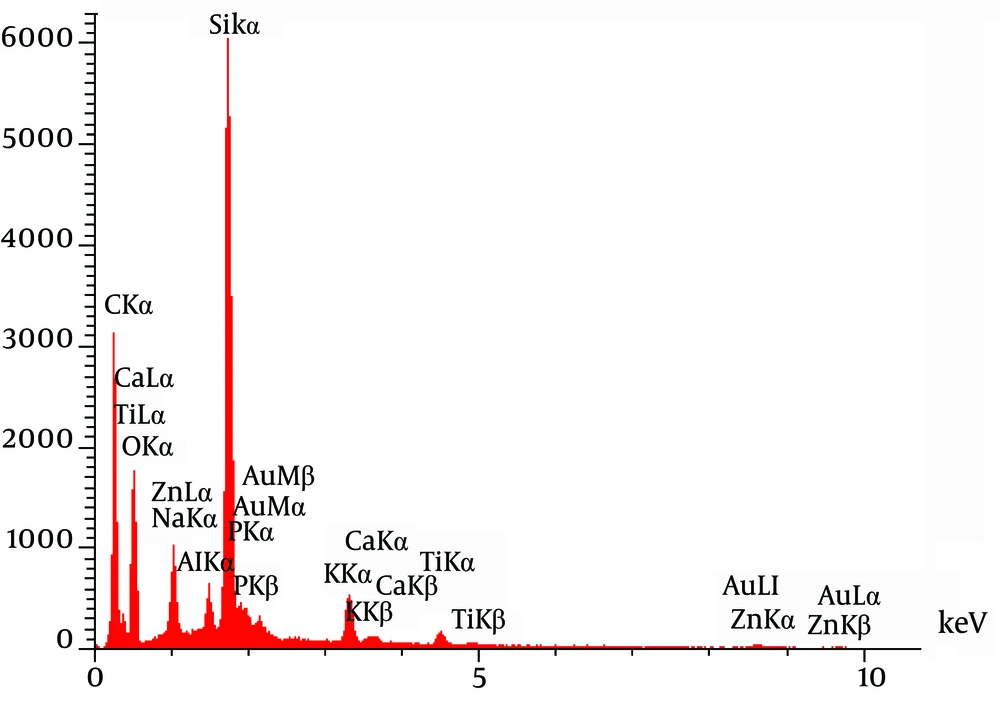
The investigation of CNPs growth was performed via changes in the optical properties of the CNPs once every 10 days of incubation time at the wavelength of 650 nm. The results indicated that the nanobacteria growth was too slow in 10 days of incubation. The pH variation was measured during the incubation time. The pH changed in the range of 7 to 8. To determine whether any other pathogen is present in the nanobacteria culture medium after filtering, the samples were cultured in current nutrient microbiological culture media every three days of incubation, showing no bacterial and fungal growth as the sign of contamination at 25, 30, 37, and 50°C. After about 10 days, the results obtained from inverted optical microscopy showed that the CNPs initiated a crystallization phenomenon and adhered to the bottom of the plates (Figure 1). After around two months, the samples were centrifuged and the two obtained phases of samples including the pellet and the supernatant were investigated using scanning electron microscopy. The evidence showed that the CNPs had spherical shapes. The SEM study showed a spherical shape for nanobacteria (Figure 2). In the culture without vascular plaques, no nanobacteria were observed. TEM results showed that the CNPs had a nanometer shell (Figure 3). Energy-dispersive spectroscopy (EDS) analysis of samples showed two elemental sharp peaks (carbon and oxygen) rather than other components (Figure 4).
5. Discussion
In atherosclerosis, plaques are formed inside the arteries. The plaques contain fat, cholesterol, calcium, and other substances found in the blood. Over time, plaques are hardened to narrow the arteries. Then, the flow of oxygen-rich blood is limited to the organs. Atherosclerosis can lead to serious problems, including heart attack, stroke, or even death. The existence of nanobacteria as nidi in any part of the body can lead to the accumulation of minerals. Hence, the nanobacteria precipitation in the interior of arteries as a spark can initiate reactions that eventually occlude the arteries, so caused atherosclerosis (22, 23). So far, different studies have been conducted on nanobacteria as a controversial issue. Confirming the results obtained from previous research, our results showed that nanobacteria were found in most extracted specimens from patients with atherosclerosis. Based on previous investigations and the results of the current study, minerals are accumulated on the cell membrane by nanobacteria to ultimately create hard apatite shells (7, 24). The ability to create these structures was confirmed using some approaches such as monoclonal antibody and turbidity assays. In the current study, we observed that the optical absorption increased with increasing the size of white sedimentations (containing nanobacteria based on SEM micrographs). Moreover, our results showed that with increasing the nanobacteria incubation time, the coating thickness of these organisms increased due to the accumulation of mineral components. Their protecting cover is so impenetrable that it is not readily accessible with anti-nanobacterial agents (24). To check whether the results obtained from nanobacteria culture are not attributed to the growth of any other organisms, it is very necessary to apply two approaches in experiments. First, all components should be sterilized. For sterilization and restricting the penetration of foreign entities, we used 0.2-μm filters. Hence, we ensured that the culture medium had minimum pathogen interference. Second, the existence of any organisms should be shown by electron microscopy. In this study, no contamination was seen in microscopy and staining methods.
This study opens new opportunities for the investigation of the pathogenesis of nanobacteria in atherosclerosis. Nonetheless, achieving more details on CNPs pathological properties needs more research.