1. Background
Tuberculosis (TB) is still among the leading causes of death worldwide, especially in developing countries. There were more than 10 million new TB cases and 1.3 million deaths from TB in 2017 (1). The emergence of multidrug-resistant TB (MDR-TB) and inappropriate diagnosis and treatment services are among the important challenges for TB control strategies (1). Based on the World Health Organization (WHO), the treatment success rate for patients with MDR-TB is less than 60% [1]. Unfortunately, patients infected with drug-resistant strains of Mycobacterium tuberculosis have to endure long, toxic, and costly therapies with poor outcomes (2-8). The problem of MDR-TB is even more serious in many low- and middle-income countries.
In Iran, a country with a moderate annual burden of TB, the MDR-TB has not been yet brought under control, despite the government’s considerable efforts (9). According to the WHO report, the estimated proportion of TB cases with MDR-TB in new- and retreatment-TB cases was found to be 1.3% and 8.3%, respectively (1). Furthermore, statistical data from the Ministry of Health do not show a significant declining trend of MDR-TB among Iranian nationals (10). Thus, knowledge about drug resistance patterns of M. tuberculosis strains plays an essential role in the control and management of the disease.
2. Objectives
Although, the prevalence of MDR-TB has been demonstrated in several studies in Iran, continues monitoring of MDR-TB is needed for the management of MDR-TB. Thus, our study aimed to investigate the prevalence of drug-resistant TB in Tehran, the capital of Iran.
3. Methods
3.1. Setting and Samples
In the present study, we retrospectively analyzed 3,012 clinical samples from suspected TB patients referred to the Masoud Laboratory from April 2012 to March 2018. Clinical specimens were collected either from new- or retreatment-TB cases. The average age of patients was between 18 and 89 years and all of them had clinical signs and symptoms of TB. The Masoud Laboratory is among the main laboratories for TB diagnosis in Tehran, Iran, which is under the supervision of the Swedish Institute for Infectious Disease Control. The Ethics Committee of Shahid Beheshti University of Medical Sciences approved the study (IR.SBMU.RETECH.REC.1396.1308).
3.2. Identification of Mycobacteria
The smear, culture, and biochemical tests were performed according to the WHO protocol (11). Clinical samples were decontaminated using the N-acetyl-L-cysteine-sodium hydroxide method. All the smears were stained with Ziehl-Neelsen method. For the identification of mycobacteria, the samples were cultured in Lowenstein Jensen (LJ) medium and isolates were identified as M. tuberculosis using the niacin, catalase, and nitrate tests, as well as bacterial pigment production and growth rate (12).
3.3. Molecular Identification of M. tuberculosis
The mycobacterial DNA was extracted using the Roche DNA extraction kit according to the manufacturer’s instructions. Then, IS6110-based PCR assay was used for the molecular identification of M. tuberculosis (13).
3.4. Drug Susceptibility Testing of M. tuberculosis
The drug susceptibility testing (DST) was performed by the proportional method as described previously (14, 15). Resistance was expressed as the percentage of colonies that grew on critical concentrations of the drugs including 0.2 µg/mL for isoniazid (INH) and 40 µg/mL for rifampicin (RIF). Mycobacterium tuberculosis H37Rv strain (ATCC 27294) was used for quality control testing in DST.
3.5. DNA Sequencing
The DNA sequencing of genes katG and rpoB was conducted to confirm the results as described previously (15).
4. Results
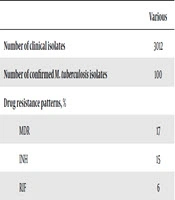
Of 3,012 clinical specimens, 100 (3.3%) were culture-positive and assigned as M. tuberculosis by phenotypic and molecular methods. Among the investigated M. tuberculosis isolates, 17 were MDR (Table 1). Based on DNA sequencing analysis, drug-resistant strains had a mutation in their hot spot region.
| Values | |
|---|---|
| Number of clinical isolates | 3012 |
| Number of confirmed M. tuberculosis isolates | 100 |
| Drug resistance patterns, % | |
| MDR | 17 |
| INH | 15 |
| RIF | 6 |
Drug Resistance Patterns of Mycobacterium tuberculosis Isolates
5. Discussion
The relatively high prevalence of MDR-TB (17.0%) makes this study relevant to all physicians treating patients with TB. The rate of MDR-TB in the current study was higher than those of previous studies from Iran. The rate of MDR-TB in studies conducted in different cities of Iran ranged from 1% to 26% (16-22).
The history of incomplete TB treatment, inadequate supply of drugs, lack of treatment supervision, and inappropriate intake of prescribed anti-TB drugs are among the critically important factors contributing to acquiring MDR-TB (23). If MDR-TB arises from incomplete treatment, improving treatment adherence can prevent further spread of resistant forms of TB (23).
In Iran, patients with MDR-TB are placed on the MDR-TB treatment regimen based on either DST results or the history of drugs taken, for a minimum of 12 months (24). The regimen usually contains an injectable aminoglycoside, a quinolone, para-aminosalicylic acid, prothionamide, pyrazinamide, ethambutol, etc. (24). However, prolonged therapy often results in a low TB treatment completion rate. To deal with this problem, some countries are evaluating other treatment regimens given for 9 - 12 months to shorten the duration of MDR-TB therapy (25-27).
The treatment success rate of MDR-TB is low in Iran, as well. This can be due to the following important factors: unavailable treatment regimens in many parts of the country, high treatment costs, ineffective treatment management and finally, limited numbers of equipped laboratories with DST capability. Thus, better treatment management and using new technologies can improve the current situation.
One of the key limitations of this study is that the information regarding the previous TB treatment was not available; thus, the prevalence of MDR-TB could not be separately analyzed in new cases or previously treated TB cases.
In conclusion, there was a high rate of MDR-TB in the current study. Improving the diagnosis and management of MDR-TB should be highly prioritized.