1. Background
Neonatal sepsis is a life-threatening condition that may lead to mortality if not treated promptly and appropriately (1). It is the main cause of infant mortality in developing countries (2). Despite vast improvements in labor management and the introduction of broad-spectrum antibiotics, still, sepsis claims many lives even in developed countries (3).
Neonatal sepsis is a clinical syndrome characterized by the signs and symptoms of infection associated with positive blood culture (1, 4). On-time diagnosis and identification of pathogens are important (5). Symptoms of neonatal infections are nonspecific and include lethargy, fever, restlessness, respiratory distress, poor feeding, cyanosis, pallor, hypothermia, vomiting, tachycardia, drowsiness, and abdominal distension (6). In a previous study, 25.46% of blood culture samples from infants were positive, among which, Gram-negatives (61.8%) were more common compared to Gram-positive (38.2%). Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae have been reported as the most common Gram-negative bacteria. Staphylococci are reported as the most resistant against penicillin. Enterobacteriaceae were also penicillin-resistant while sensitive to norfloxacin, amikacin, and tobramycin; therefore, norfloxacin was the most effective antibiotic against Gram-negative bacilli in pediatrics wards (3).
Bacteria are the most important causes of sepsis. Fungi, viruses, and parasites showed to have a less important role in neonatal infections. However, they should not be ignored (7). Untreated blood infections may cause more dangerous conditions, multi-organ involvement, and finally, death. The spectrum of the microorganisms responsible for blood infections varies in different countries and even within the different districts of the same country. However, Gram-negatives have a bigger share, especially in Asian countries (8).
Choosing the right antibiotic in suspected infections is often a challenging task for neonatalogists. Identifying bacteria colonized in the maternal genital tract (in early sepsis) and the prevalent microorganisms in NICU, including on personnel' hands, as well as their sensitivity to the antibiotics used in the NICU, is of crucial importance. Little information is available about the sensitivity and resistance of antibiotics for both Gram-positive and Gram-negative bacteria in neonatal sepsis. Neonatal sepsis is a life-threatening condition, mainly due to the lack of proper and on-time treatment. Determining the antibiotic sensitivity pattern is essential for the proper treatment of neonatal sepsis (9).
2. Objectives
In this large retrospective study, we have investigated the sensitivity and resistance of Gram-positive and Gram-negative bacteria of definitive neonatal sepsis to various commercially available antibiotics in Ghaem Hospital, Mashhad, during a period of 10 years (2009 to 2019).
3. Methods
In this cross-sectional study, of 5426, neonates referred to the NICU of Ghaem Hospital, Mashhad, from 2009 to 2019, 268 cases with definitive neonatal sepsis due to Gram-positive or Gram-negative germs are enrolled. The study was approved by the research committee of the Mashhad University of Medical Sciences (IR.MUMS.fm.REC.1396.587. Code: 960925).
Neonates without enough follow-up, patients with missing antibiogram results in hospital records, and those who left the hospital before the completion of the examinations were excluded from the study. A researcher-made questionnaire, including patients' characteristics, was used to collect data. The content validity of the questionnaire was verified by five faculty members in the School of Medicine affiliated with the Mashhad University of Medical Sciences. Age at onset of sepsis, symptoms, signs, duration of hospitalization, diagnosis, and treatment, as well as complete neonatal examination, were determined. Afterward, laboratory tests for the diagnosis of sepsis were performed. The signs and symptoms of neonatal infections included fever, lethargy, restlessness, poor feeding, respiratory distress, cyanosis, pallor, hypothermia, vomiting, tachycardia, and abdominal distention (10).
For bacterial isolation and identification, the specimens were cultured according to standard methods. Antimicrobial susceptibility testing (AST) is a laboratory procedure performed by medical technologists (clinical laboratory scientists) to evaluate the effectiveness of antimicrobial regimens for individual patients.
Culture media were incubated at 37°C for 18 hours under sterile circumstances. Disc diffusion susceptibility test was performed using antibiotic discs. The results were reported after 18 hours of incubation at 37°C, according to the Clinical Laboratory Standards Institute (CLSI) (10). Data were analyzed using SPSS v.16. The mean, standard deviation, and frequency tables were used to describe the data.
4. Results
As blood culture takes time, empirical antibiotic therapy is a commonly used practice; however, many germs present different degrees of resistance and sensitivity to empirical antibiotic therapy (Figure 1). In this study, the blood cultures of 268 infants were positive. Positive first blood culture was reported in 58 cases, while 201 cases were positive in the second culture, 7 cases before and 2 cases after exchange transfusion. Blood culture reported both Gram-negative (176 infants) and Gram-positive bacteria (92 infants).
4.1. Gram-Negative Bacteria
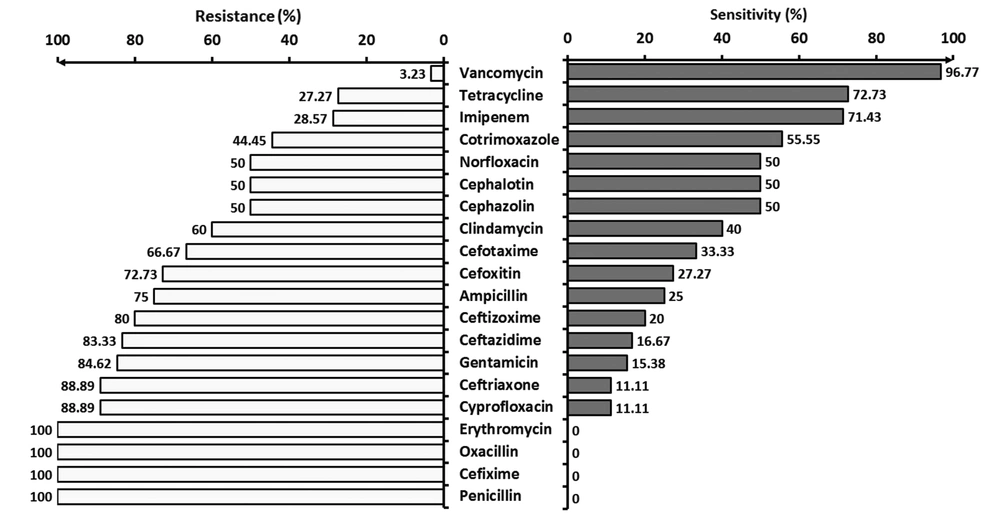
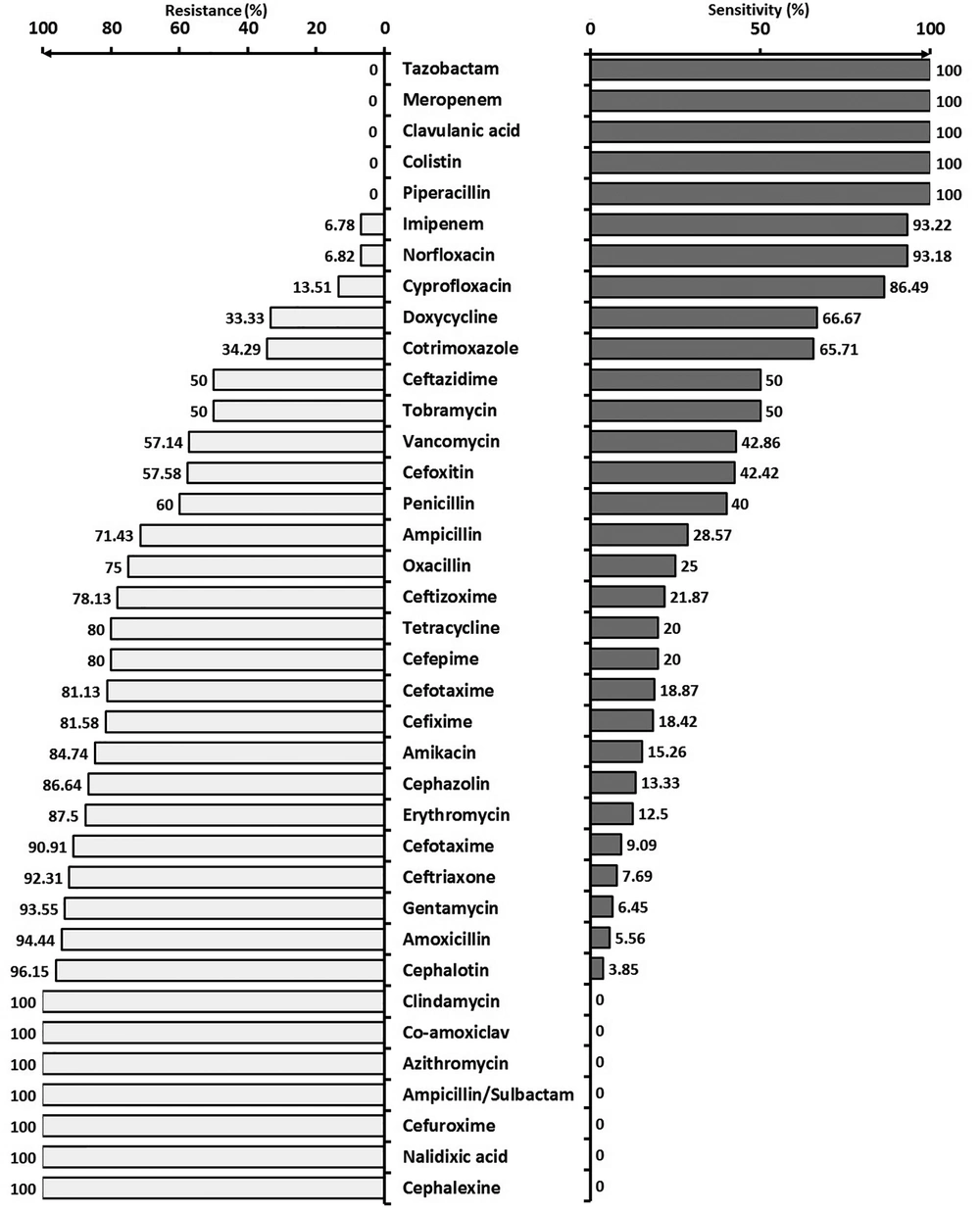
Figure 2 shows the resistance and sensitivity of Gram-negative bacteria to various commonly used antibiotics.
4.2. Gram-Positive Bacteria
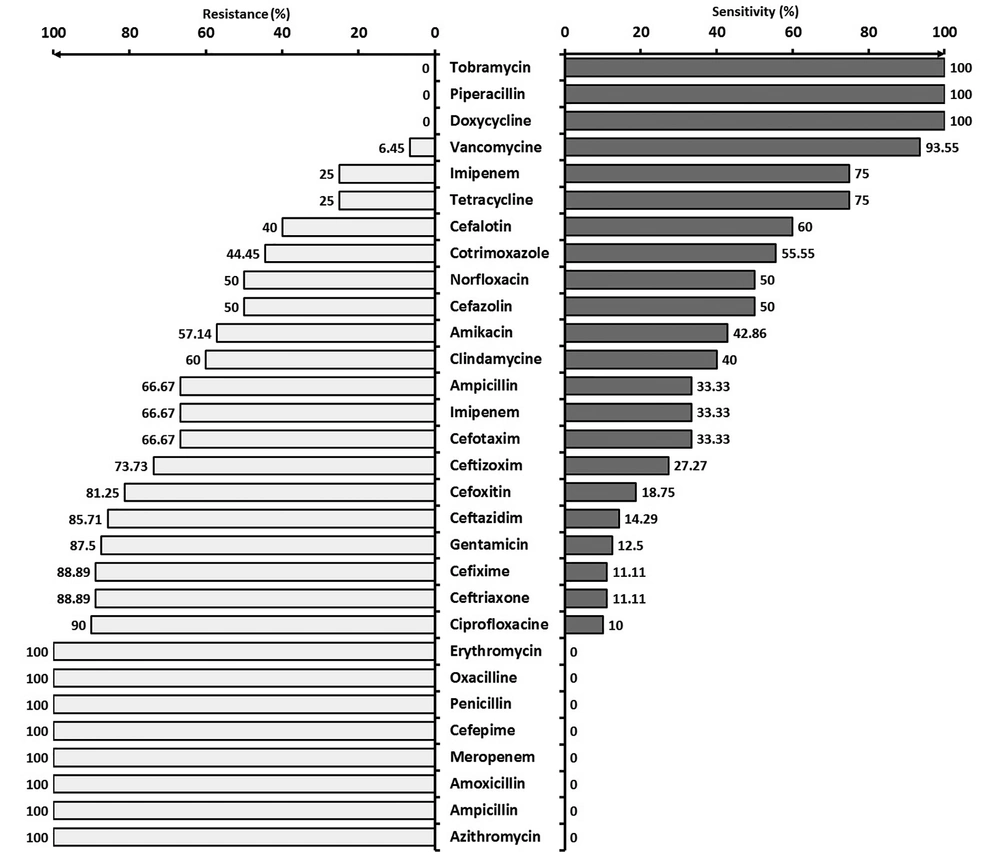
Figure 3 shows the resistance and sensitivity of Gram-positive bacteria to various commonly used antibiotics.
5. Discussion
Despite the introduction of various broad-spectrum antibiotics, the sensitivity and resistance of different microorganisms responsible for infantile sepsis to the commercially available antibiotics are still unclear. In this study, the sensitivity and resistance of the bacteria responsible for neonatal sepsis to various antibiotics were evaluated. According to our findings, Gram-negative germs were responsible for 2/3 of definitive infant sepsis cases, while the rest of the infections were caused by Gram-positives.
As a limitation of the current study, although the responsible germs have been grouped as Gram-negatives and Gram-positives, they have not been differentially defined.
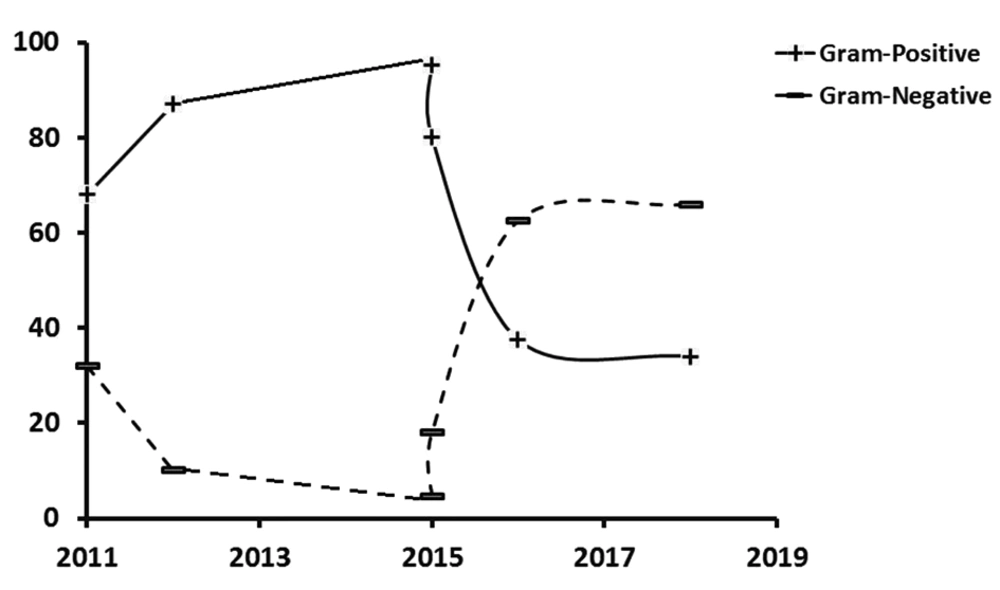
The prevalence of Gram-negative and Gram-positive bacteria during the last seven years (that is, from 2011 to 2017) had a biphasic crossover trend, as reported by the studies in Turkey, Iran, and India (2, 4, 11-14). While Gram-positives showed a growing trend, Gram-negatives have been regressing until 2015, where they started reversing, and by 2018 Gram-negatives reached 66%, and Gram-positives dropped to 34% of all germs responsible for infantile sepsis (Figure 4). In a previous study conducted in 2015 in southern Iran, less than 5% of the bacteria in blood cultures were found to be Gram-negatives, mostly from the emergency and internal departments as well as the ICU. They reported Escherichia coli, Pseudomonas aeruginosa, Acinetobacter, and Klebsiella as the most common Gram-negatives that were resistant against must antibiotics. The highest resistance was found against ceftazidime and ceftriaxone, while ciprofloxacin and imipenem showed the least resistance (13). However, a simultaneous report from India showed a high sensitivity of Gram-negative organisms to imipenem, meropenem, and piperacillin/tazobactam (14).
The study by Prabhu's reported that Gram-negative organisms had the highest resistance (64.28%) against ampicillin. Similar to other members of the Enterobacter family, a high resistance (53.8%) was found to the third-generation cephalosporins (15). The high prevalence of Gram-negative organisms in the present study and other similar studies can be attributed to the overcrowding of hospital departments, high bed-to-nurse ratios, and lack of proper hand hygiene.
Out of 100 infants suspected of late sepsis in the study by Rafati et al., 20 had positive blood cultures with Staphylococcus aureus (35%), followed by Klebsiella pneumoniae (20%) and Escherichia coli (20%), which were the most common germs (2). Sensitivity loss of the bacteria responsible for neonatal sepsis to the current antibiotics necessitates their rational prescription and consumption (3).
According to the findings of Behjati et al., coagulase-positive and negative staphylococci among Gram-positives; and Klebsiella and Entrobacter among Gram-negatives have been the most common pathogens. Although ampicillin and gentamicin have been recommended by most textbooks as the first drugs of choice for infantile sepsis, high resistance was reported to ampicillin and gentamicin (35 - 75% of cases). Therefore, ampicillin and gentamicin can no more be prescribed when these germs are suspected (16). Mutlu et al. in Turkey reported that Carbapenem was the drug of choice for nosocomial infections caused by Gram-negative bacteria in NICU (11).
Gram-positive bacteria were completely sensitive to piperacillin, and tobramycin. They also showed high sensitivity to vancomycin (93.55%), imipenem (75%), and tetracycline (75%). Gram-positives were sensitive to co-amoxiclav in 2/3; clindamycin in 1/3; and amikacin in 4/5 of the cases. They were relatively sensitive to cefazolin, norfloxacin, and co-trimoxazole. Gram-positive bacteria were quite resistant to azithromycin, ampicillin, gentamicin, cefepime, meropenem, penicillin, erythromycin, oxacillin, and amoxicillin. In another study, 70% of the Gram-positive microorganisms were shown to be resistant to penicillin, and 90% of the Gram-negatives were resistant to gentamicin and ampicillin (17). According to a different study, all Gram-positive bacteria showed 100% sensitivity to vancomycin, theicoplanin, and rifampicin (14). In Prabhu et al.'s study, 63.4% of Staphylococcus aureus species were resistant to penicillin. Penicillin-resistant Staphylococcus aureus colonies were treated by cloxacillin, methicillin, and naficillin. Reducing the sensitivity of infectious septic bacteria to common antibiotics depends on their rational prescription and consumption (15).
In a study by Rafati et al., the resistance of Gram-negative bacteria to ceftazidime, cefotaxime, ceftizoxime, and ceftriaxone was 72.7%, 54.5%, 54.5%, and 60%, respectively. All (100%) Gram-positives are reported to be resistant to oxacillin and 77.7% to cephalotin and clindamycin. The most effective antibiotics against Gram-positive and Gram-negatives were vancomycin and imipenem (100% sensitivity), respectively. Therefore, resistance to cephalosporins has increased, and hence, their prescription must be reconsidered (2).
The microorganisms in neonatal wards have shown very good sensitivity to vancomycin (97%), an acceptable sensitivity to imipenem (72%), and a relative sensitivity (~50%) to co-trimoxazole, norfloxacin, cephalotin, and cefazolin. About two-thirds of the cases were resistant to ampicillin, clindamycin, cefotaxime, and cefoxitin. About four-fifths of the microorganisms in our neonatal ward were resistant to gentamicin, ceftizoxime, and ceftazidime. All microorganisms in our NICU were resistant to erythromycin, oxacillin, cefixime, and penicillin. In a study by Shrestha et al., all microorganisms in the NICU, except Acinetobacter, were sensitive to the first-line antibiotics (e.g. amikacin, gentamicin, cefotaxime, and ampicillin) (18). The high resistance of microorganisms in our NICU to the first-line antibiotics, such as ampicillin and Gentamycin, is a serious problem and can be due to the unusual use of these agents. On the other hand, according to the high resistance of these drugs, it seems that the onset of these antibiotics will not be effective in treating infants with a high risk of infection, and revision of these two drugs seems necessary. The inappropriate sensitivity of microorganisms to cephalosporins, which usually are the second choice antibiotics in our NICU, is the second worrying result.
In general, the pathogens responsible for neonatal septic are different over time and even from place to place (19). Antibiotic resistance is a global problem. The pattern of antibiogram varies from country to country depending on the epidemiology of neonatal sepsis (20). The difference in the patterns of antibiotics used in different hospitals is the main cause of various antibiotic sensibility reported by different researchers (21).