1. Background
Mycobacterium tuberculosis (TB) is a frightening infectious disease affecting nearly one-third of the world’s population (1). Although pulmonary TB is the most common clinical subtype, TB can also orchestrate multi-organ dysfunction by invading extra-pulmonary tissues. Tuberculosis is also a major health concern in Iran. Especially, an increased rate of drug-resistant infections has been encountered in recent years in the country (1). Tuberculosis is particularly common in the South-Eastern Regions of Iran (2). Accordingly and with an estimated rate of 3.6% and annual incidence rate of 0.48%, Sistan and Baluchestan Province is an endemic region of TB in Iran (3).
Infectious diseases have been noted as potential culprits in the development of autoimmune conditions (4). Accordingly, patients infected with mycobacterial infections have been detected with higher susceptibility to autoimmune conditions and the emergence of autoantibodies (5-8). A relationship has been noted between celiac disease (CD) and TB (9-11). Celiac disease is a common autoimmune etiology of intestinal atrophy. Generally, CD is diagnosed as a cause of malnutrition in 1% of Mediterranean populations (12). Association of CD with genetic determinants has been well stablished during years of extensive research. In particular, CD has been associated with human leukocyte antigen (HAL)- DQ2 and DQ8 haplotypes (13, 14). Autoimmunity against intestinal enterocytes in CD is promoted by gluten (i.e. gluten triggered enteropathy) and is mediated by IgA antibodies targeting tissue transglutaminase (tTG). Serological evaluation of anti-tTG IgA antibody is the most common test used for CD screening (12, 14). Celiac disease is usually alleviated by administrating gluten-free diet; however, there is a necessity for screening other malnutrition conditions in non-responders (15).
2. Objectives
The association of TB with the development of autoantibodies necessitates routine screening of these patients and divulging the clinical importance of these antibodies in the clinical course of TB. There was no study on the clinical implications anti-tTG IgA antibody in TB affected individuals. Considering that CD is a common clinical condition in Sistan and Baluchestan Province of Iran (16), we here aimed to screen anti-tTG IgA antibodies in patients with TB in this region.
3. Methods
3.1. Patients
This was a cross sectional study performed during November 2017-June 2018. The study was performed on patients with confirmed smear-positive TB diagnosed in the Respiratory Diseases Management Center of Zabol. The diagnoses had also been approved by chest radiography. The diagnosis and clinical management of these patients had been performed according to the national guidance on diagnosis and management of TB (17).
All the eligible patients registered at the center were enrolled by accessible sampling method. Informed consent was acquired from the patients before gathering their data. The study was approved by the local Ethical Committee of Medical Researches of Zabol University of Medical Sciences.
3.2. Measuring Anti-tTG IgA
Blood samples (3 mL) were drawn in fasting condition. The samples were immediately transferred to the laboratory of the center for separating sera. The serum samples were then stored at -80°C until use. The level of anti-tTG IgA antibody was determined using a specific ELISA kit (Pars Azmoun, Iran) as noted in our previous study (18). Antibody titer > 18 IU/L was considered as positive.
3.3. Statistical Analysis
Statistical methods were performed in SPSS 19 software. Kolmogorov-Smirnov test was applied for checking the data distribution. Univariate analyses were applied as independent samples student t-test (normally distributed quantitative variables) and Mann-Whitney U test (non-normally distributed quantitative variables, i.e. anti-tTG titer). The P value < 0.05 was considered as statistically significant.
4. Results
From a total of 162 recruited patients; 100 (61.7%) were married, 30 (18.5%) were widowed or divorced and 32 (19.8%) were single. Females and males constituted 87 (53.7%) and 75 (46.3%) respectively. The mean age was 51.7 ± 22.3 years old (range of 1 - 83). The means of height and weight were 160 ± 17.8 cm and 50.8 ± 14.4 kg respectively. Afghan patients constituted 16 (9.9%) and the remaining were Iranians.
The therapy course was successfully completed in 78 (48.1%) patients, and 67 (41.4%) of them showed improved symptoms following treatments. Eight patients (4.9%) died of disease-related complications. Three patients (1.9%) did not receive any treatment, 3 (1.9%) were currently under treatment, and 2 (1.2%) did not respond to the treatments. One (0.6%) patient was incorrectly diagnosed as TB. A history of contact with a person diagnosed with TB was recorded in 47 (29%).
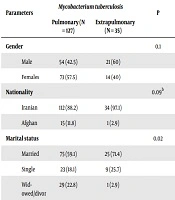
Pulmonary TB was diagnosed in 127 (78.4%) while 35 (21.6%) had extra-pulmonary disease. The mean titer of anti-tTG antibody was 22.59 ± 107.7 IU/mL (range of 0.8 - 940). The mean titers of anti-tTG IgA antibody were 21.9 ± 112.7 IU/mL and 24.8 ± 87.9 IU/mL for pulmonary and extrapulmonary TB, respectively (P > 0.05, Table 1). Overall, 19 (11.9%) of the patients showed positivity for anti-tTG. Seven patients had borderline results (titer of 12 - 18). In replication, they rendered negative results summing up the negative cases to 143 (88.1%). There was no significant association between anti-tTG positivity with neither demographic nor clinical variables (Table 2).
| Parameters | Mycobacterium tuberculosis | P | |
|---|---|---|---|
| Pulmonary (N = 127) | Extrapulmonary (N = 35) | ||
| Gender | 0.1 | ||
| Male | 54 (42.5) | 21 (60) | |
| Females | 73 (57.5) | 14 (40) | |
| Nationality | 0.09b | ||
| Iranian | 112 (88.2) | 34 (97.1) | |
| Afghan | 15 (11.8) | 1 (2.9) | |
| Marital status | 0.02 | ||
| Married | 75 (59.1) | 25 (71.4) | |
| Single | 23 (18.1) | 9 (25.7) | |
| Widowed/divorced | 29 (22.8) | 1 (2.9) | |
| History of contact with infected person | 0.1 | ||
| Yes | 34 (26.8) | 13 (37.1) | |
| No | 93 (73.2) | 22 (62.9) | |
| Age | 55.9 ± 21.4 | 36.5 ± 18.9 | < 0.001 |
| Weight | 49.2 ± 13 | 56.3 ± 17.5 | 0.01 |
| Anti-tTG IgA, IU/mL | 21.9 ± 112.7 | 24.8 ± 87.9 | 0.4c |
aValues are expressed as No. (%) or mean ± SD.
bFischer exact test.
cMann-Whitney U test.
| Parameters | TB Status for Anti-tTG | P | |
|---|---|---|---|
| Negative < 18 (N = 143) | Positive > 18 (N = 19) | ||
| Gender | 0.9 | ||
| Male | 64 | 10 | |
| Females | 76 | 9 | |
| Nationality | 0.4 | ||
| Iranian | 126 | 18 | |
| Afghan | 15 | 1 | |
| Marital status | 0.5 | ||
| Married | 86 | 13 | |
| Single | 29 | 3 | |
| Widowed/divorced | 26 | 3 | |
| Disease location | 0.7 | ||
| Pulmonary | 112 | 14 | |
| Extra-pulmonary | 29 | 5 | |
| History of contact with infected person | 0.1 | ||
| Yes | 45 | 2 | |
| No | 96 | 17 | |
| Age | 52.7 ± 21.3 | 0.8 | |
| Weight | 53.8 ± 11.5 | 0.3 | |
aValues are expressed as mean ± SD.
5. Discussion
We hereby have assessed a potential relationship between TB clinical course and seropositivity for anti-tTG IgA antibody. Overall, 19 (11.9%) patients rendered seropositivity for anti-tTG IgA antibody. However, there were no significant associations between the seropositivity for anti-tTG IgA and either demographic or clinical features. There was no significant difference in the antibody titer between pulmonary and extra-pulmonary TB.
An elevated risk of TB as high as 2-fold has been reported in biopsy verified CD patients, especially within the first year (11). TB itself can present as an intestinal disease mimicking a malnutrition status (19). In fact, intestinal TB may be misdiagnosed as a malabsorption syndrome (20-26). In one study, 13% and 9% of severely malnourished children were diagnosed with CD and TB respectively (27). The common genetic signature at HLA class II locus can be causative for both CD (as an autoimmune entity) and TB (as an inflammatory disease) (28). Furthermore, the HLA-B8 allele has been reported as a risk factor for both TB and CD (29). In this study, although 19 out of 162 (11.9%) TB patients showed anti-tTG seropositivity, no one revealed clinical symptoms of CD. Nevertheless, we could not rule out this condition without intestinal biopsy. Overall, clinically insignificant elevation of anti-tTG IgA antibody without overt CD in the course of TB should be carefully monitored by physicians.
Infection-induced alterations in immunoregulatory processes can potentially be associated with aberrant responses against auto-antigens. The risk of autoimmunity in TB patients can be influenced by genetic variations in immune system genes such as IFN-γ, and CD 14 (30-33). The role of Th-17 lymphocytes is yet to be explored as a possible dominant modulator of inflammatory autoimmunity in the context of TB infection (34).
It seems that antibodies against TB derived proteins such as heat shock protein 65 (Mt-Hsp65) (35, 36) and chaperonin 10 (m-Cpn10) (37) can promote cross-reactions with human antigens triggering autoimmunity in this condition (38). Also, immune reactions against Mt-Hsp70 has been shown to activate T cell-medicated autoimmunity (39). Identification of antigens that can promote antibody cross-reactions can assist to better understand the pathogenesis of TB and its potential associations with autoimmune conditions.
An interaction between CD and TB diseases can be assumed by some evidence from researches. As a malnutrition status, CD can be associated with deficiencies in multiple micro-nutrients and vitamins, in particular vitamin D, both as a result of general malabsorption and vitamin-deficit regimens (40). Vitamin D has a crucial role in augmenting immune responses against microbial pathogens and suppressing the growth of intracellular microorganisms such as TB within phagocytes (41). Furthermore, TB infection may contribute to CD by inducing gluten-sensitivity through accelerating the transportation of gluten across the intestine (11). The altered biology and distribution of drug metabolizing enzymes in the gastrointestinal wall of CD patients can further modulate the efficiency of medications in TB (42). Accordingly, Shetty and McKendrick (43) reported two patients with pulmonary TB who did not respond to treatment while both of them suffered from co-existing CD. In our study, two non-responder patients had anti-tTG titers of 1.1 IU/L and 5.3 IU/L respectively; negating this hypothesis. This indicates that treatment response in TB is a multifactorial phenomenon and under influence of other potential molecular and environmental factors.
In this study, we had no access to details on therapeutic regimens, the duration of treatments and follow-up, clinical features of those in whom treatment failed, and other relevant clinical data. Nevertheless, the number of anti-tTG positive cases did not get high enough to allow us for a good statistical analysis on the relevance of these clinical variables. In any future study, we recommend providing a complete clinical picture of TB patients and its potential association with anti-tTG positivity.
5.1. Conclusions
According to our observation, seropositivity for anti-tTG IgA antibody is relatively common in patients with TB. Although we found no association between this serologic marker and TB clinical parameters, it is advisable to further explore the implication of this observation on the clinical course of TB patients.