1. Background
Leptospirosis is a common zoonosis disease, caused by various Leptospira species belonging to the spirochaete family. Leptospira spp. may enter the host through an abraded skin or intact mucous membranes. Although the organism mainly infects the kidneys and liver, any organ may get involved. Today, culture or serological methods such as immunofluorescence assay (IFA), ELISA, microscopic agglutination test (MAT), and molecular methods based on polymerase chain reaction (PCR) are used to diagnose Leptospira spp (1-5). Leptospirosis is vasculitis with systemic involvement and has spirochetal and safety phases and can create subacute syndrome; the mild and jaundice-free cause symptoms such as flu, which their differentiation from other fever-like illnesses is difficult. Moreover, it worth noting that most patients do not present all the symptoms together. The jaundice form with bleeding or Weil’s syndrome that is a severe form of the disease, which involves various organs of the body and is characterized by three main symptoms of jaundice, mucosal bleeding, and acute kidney injury (AKI) (1, 4-7). Leptospirosis primary lesions include disruption of the cell membrane of the endothelial layer of small blood vessels throughout the body that causes capillary leakage and bleeding. Widespread petechial bleeding occurs in all tissues and organs. Anoxia caused by blood vessel damage in the kidney cortex leads to tubular necrosis, especially in the proximal tubules, and eventually leads to acute renal failure (8). It seems vasculitis infects more capillaries, and therefore, more symptoms of leptospirosis are present in filtering organs such as kidneys, lungs, and liver. Kidney involvement is more common in leptospirosis and accounts for the most common cause of death from the disease (8-10). New evidence suggests that activation of the endothelial destruction process in disorders of endothelial cells layer may be the most important pathophysiological mechanism in leptospirosis (10). Angiopoietin-2 is a marker of endothelial destruction process activation and systemic inflammation. The absence of vascular endothelial growth factor (VEGF) accelerates cell death and regression of coronary. Besides, along with VEGF, angiopoietin-2 can promote vascular endothelial cell growth and the formation of new vessels. Increased levels of angiopoietin-2 enhance the number of new blood vessels in tumors, metastasis, and inflammation. Angiopoietin-2 role in inflammation and cancer should have clinical value. Angiopoietin-2 can be used as an indicator to determine the severity of the disease in critically ill patients. Despite the availability of this indicator, it is not yet in routine clinical use. Bacterial toxins such as lipopolysaccharide (LPS) can be a powerful stimulus for the release of angiopoietin-2 (11-18).
2. Objectives
Considering the high prevalence of leptospirosis in Mazandaran Province and also considering that leptospirosis may even result in the death of the patient at an advanced stage, this study aimed to determine the value of angiopoietin-2 biomarker in predicting leptospirosis complications in people with the disease and preventing dangerous complications of this disease.
3. Methods
This study is a prospective observational study conducted on patients with leptospirosis admitted at Razi Hospital in Qaemshahr, Mazandaran Province of Iran (a teaching hospital for infectious diseases). This study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (code no.: 1578). Besides, written informed consent was obtained from participants.
For confirming leptospirosis, the serum sample of patients suspected leptospirosis was tested by the IFA method, if the test was negative, the patient’s sample was taken again, and the test was repeated by MAT method. Any antibody titer greater than 1:800 was considered positive (4).
Inclusion criteria were being older than 18 years of age and having an infectious syndrome suspected to leptospirosis. Exclusion criteria were refused informed consent, cases with a negative result by IFA and MAT test, patients who were hospitalized with symptoms of sepsis one month before the visit time, and patients with liver or kidney diseases.
All patients were fully examined by an infectious disease specialist. The demographic data such as age, sex, occupation, residence, place of disease, and the contact with contaminated water along with the onset of symptoms, clinical symptoms, duration of hospital stay, probable complications, and mortality according to the patient’s statements and frequent examinations recorded were collected.
Totally 5 mL venous blood was taken from patients with suspected leptospirosis 3 to 5 days after the onset of symptoms and before starting treatment with antibiotics according to World Health Organization criteria (presence of acute febrile illness with fatigue, headache, myalgia, and one of the symptoms of conjunctival congestion, signs of meningeal irritation, oliguria or anuria (defined as the urinary output of < 500 mL and 100 mL, 24 h, respectively), proteinuria, jaundice, bleeding, arrhythmias or heart failure, skin rash). After being centrifuged, the blood plasma was separated and kept at -80°C until the time of measuring the angiopoietin-2 level. The levels of angiopoietin-2 in serum were measured by ELISA methodology [R & D Systems; Minneapolis, MN, US] after obtaining the second sample (5 mL venous blood) 10 to 14 days after the onset of symptoms. Also, a control group that was homogenous with the experimental group in terms of age and gender was enrolled in the study to compare the angiopoietin-2 level in the patient group, and also of 5 mL of venous blood was taken from this group. A complete blood count (CBC) test and biochemical tests including counting the mean of hemoglobin, platelets, blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), C-reactive protein (CRP), Bill direct, Bill total, and urine analysis were performed. Finally, the serum levels of angiopoietin-2 and its relationship with symptoms and complications of leptospirosis were analyzed using statistical methods by SPSS Ver.16. Descriptive statistical methods were first used to analyze data. Mean ± standard deviation (SD) and Q2 (Q1-Q3) relationships were used for quantitative variables with normal distribution and quantitative variables with non-normal distribution, respectively. Also, frequency tables were used for qualitative variables. Data analysis was performed using inferential statistics, GLM Generalized Linear Models, and Rock charts were used to calculate diagnostic value and sensitivity, specificity, and positive and negative predictive value and positive likelihood ratio. Also, to compare two groups based on the assumption of normality, the Student’s t-test was used according to the Shapiro-Wilk test results. Also, data were converted using a Box-Cox in case no assumption of normality and if data were still non-normal, Mann-Whitney was used. The Youden test was used to determine the cut point.
4. Results
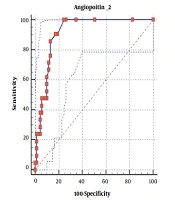
In total, 90 individuals (equally distributed into two groups of patients and controls) involved in the study. The results revealed that men (75% or 83.3%) accounted for a greater gender proportion compared with women (15% or 16.7%). The minimum and maximum age range of participants were 23 and 75, respectively. Most patients with leptospirosis were employed in agriculture (74% or 82.3%) and the majority of them lived in rural areas (59% or 65.6%). Demographic characteristics were recorded for case and control groups (Table 1). Frequency, symptoms, complications, and laboratory findings, as well as clinical characteristics of participants, are provided in Tables 2 - 4. According to the results obtained from the collected sample, it can be concluded that the most frequent complication was anemia (29 patients, 32.2%). Table 4 shows the ROC curve analysis used to determine the diagnostic value of angiopoietin-2 biomarker, the area under the curve (AUC) of this biomarker, and P-value and confidence intervals. ROC curve of angiopoietin-2 biomarker is shown in Figure 1. Values of diagnostic value indices (sensitivity, specificity, positive and negative predictive value, and positive likelihood ratio) and 95% confidence intervals are entered in these tables.
| Variables | Case | Control | Total |
|---|---|---|---|
| Gender | |||
| Male | 38 (84.4) | 37 (82.2) | 75 (83.3) |
| Female | 7 (15.6) | 8 (17.8) | 15 (16.7) |
| Job | |||
| Other | 5 (11.1) | 11 (24.4) | 16 (17.7) |
| Farmer | 40 (88.9) | 34 (75.6) | 74 (82.3) |
| place of living | |||
| City | 13 (28.9) | 18 (40) | 31(34.4) |
| Village | 32 (71.1) | 27 (60) | 59 (65.6) |
| Direct contact with water. | |||
| Yes | 45 (100) | 0 (0) | 45 (50) |
| No | 0 (0) | 45 (100) | 45(50) |
| Place of infection | |||
| Rice field | 44(97.8) | 45 (100) | 89 (98.9) |
| Swim | 1 (2.2) | 0 (0) | 1 (1.1) |
| Age | |||
| < 40 | 12 (26.7) | 36 (80) | 48 (53.3) |
| ≥ 40 | 33 (73.3) | 9 (20) | 42 (4.7) |
Demographic Characteristics of Participants Separated by Groupa
| Variables | Frequency (%) |
|---|---|
| Fever | |
| Admission | 43 (95.6) |
| During hospitalization | 18 (40) |
| Headache | |
| Admission | 41 (91.1) |
| During hospitalization | 30 (66.7) |
| Muscular pain | |
| Admission | 45 (100) |
| During hospitalization | 30 (66.7) |
| Conjunctival hyperemia (Suffusion) | |
| Admission | 19 (42.2) |
| During hospitalization | 14 (31.1) |
| Beech | |
| Admission | 2 (4.4) |
| During hospitalization | 2 (4.4) |
| Yellow | |
| Admission | 7 (15.6) |
| During hospitalization | 8 (17.8) |
| Cough | |
| Admission | 9 (20) |
| During hospitalization | 8 (17.8) |
| Symptoms of meningeal irritation | |
| Admission | 0 (0) |
| During hospitalization | 0 (0) |
| Shortness of breath | |
| Admission | 8 (17.8) |
| During hospitalization | 4 (4.9) |
| Pulmonary infiltration | |
| Positive | 3 (6.7) |
| Negative | 42 (93.3) |
| Hemorrhage | |
| Positive | 0 (0) |
| Negative | 90 (100) |
Frequency of Symptoms of Leptospirosis
| Variables | Case | Control | Total |
|---|---|---|---|
| Anemia | |||
| Yes | 26 (57.8) | 3 (6.7) | 29 (32.2) |
| No | 19 (42.2) | 42 (93.3) | 61 (67.8) |
| Thrombocytopenia | |||
| No | 5 (11.1) | 50 (0) | 55 (55.6) |
| Weak | 10 (22.2) | 0 (0) | 10 (11.1) |
| Average | 8 (17.8) | 0 (0) | 8 (8.9) |
| Leukocytosis or leukopenia | |||
| Severe | 22 (48.9) | 0 (0) | 22 (24.4) |
| Positive | 24 (53.3) | 0 (0) | 24 (26.7) |
| Negative | 21 (46.7) | 45 (100) | 66 (73.3) |
| CRP ≥ 2+ | |||
| Positive | 21 (46.7) | 0 (0) | 21 (23.3) |
| Negative | 24 (53.3) | 45 (100) | 69 (76.7) |
| Hyperbilirubinemia | |||
| Positive | 20 (44.4) | 0 (0) | 20 (22.2) |
| Negative | 25 (55.6) | 45 (100) | 70 (77.8) |
Laboratory and Clinical Findings of Leptospirosis
| Angiopoietin-2 | |
|---|---|
| AUC | 0.932 (1 - 0.85) |
| P-value | < 0.001* |
| Sensitivity | 88.2 |
| Specificity | 95.7 |
| Positive predictive value | 91.7 |
| Negative predictive value | 93.8 |
| Positive likelihood ratio | 33.2 |
| Cut-point | 8.5< |
Values of Diagnostic Criterion and ROC Curve Coordinates Based on Angiopoietin-2 Biomarker
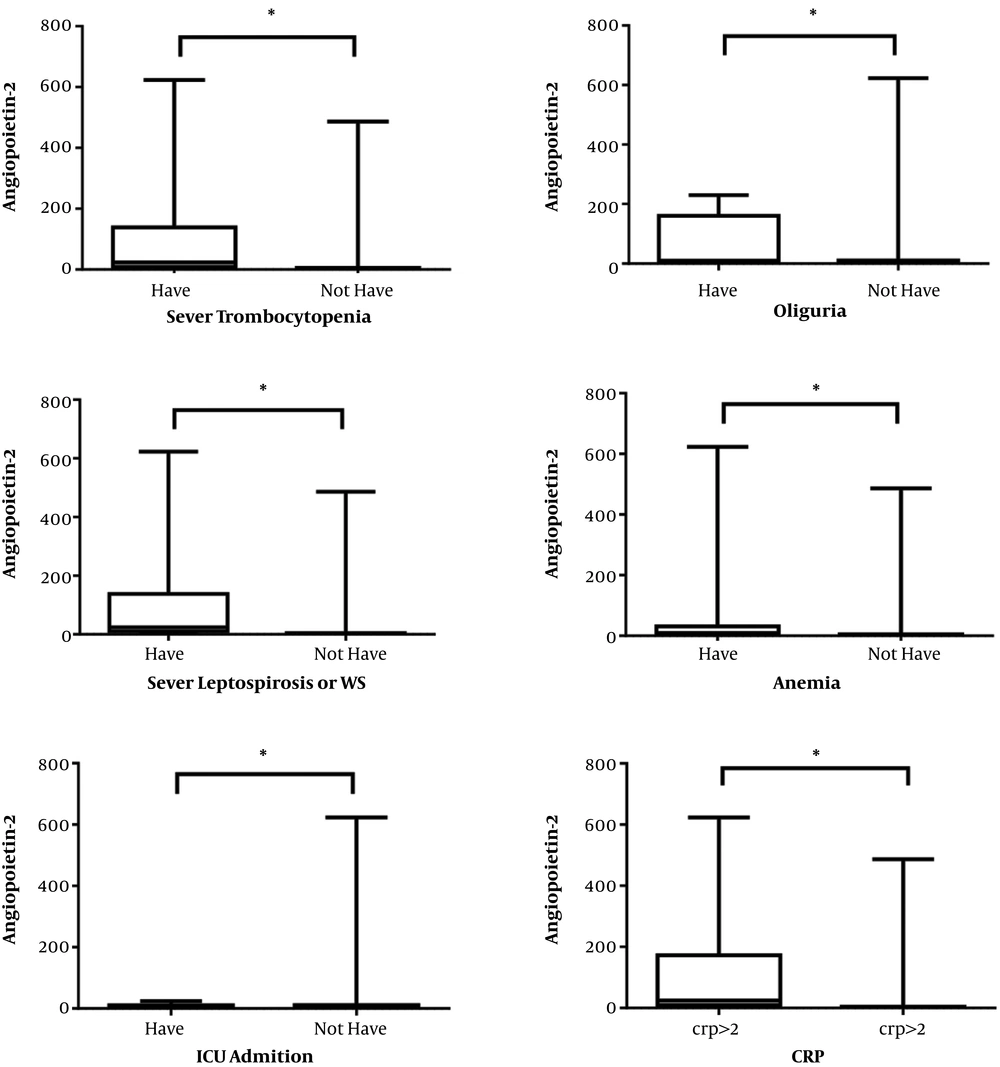
The Mann-Whitney test was used to investigate the relationship between angiopoietin-2 level and disease severity criteria based on being older than 40 years, change in LOC (admission, during hospitalization, at discharge), anemia, oliguria, anuria, hyperkalemia, pulmonary infiltration, severe thrombocytopenia, and need to ventilator. The results are shown in Table 3. The results indicated a significant relationship between the angiopoietin-2 level in case and control groups (P-value < 0.01) in the sense that greater angiopoietin-2 levels were observed in the case group. There was also a significant relationship between angiopoietin-2 and the disease severity at age groups below and above 40 years (P-value = 0.02). There was also a significant relationship between angiopoietin-2 and anemia (P-value < 0.01), so that its level was higher in people with anemia. We also found a significant relationship between angiopoietin-2 and oliguria (P-value < 0.01) and severe thrombocytopenia (P-value < 0.01). Thus, it can be concluded that elevated angiopoietin-2 levels was significantly related to leptospirosis severity. According to the Mann-Whitney test results, there was also a significant difference between the average angiopoietin-2 rating in the groups with and without CRP > 2+ (P-value < 0.001). Considering the average rating, it can be stated that the average rating of angiopoietin-2 is higher in the group with CRP > 2+. The relationship between angiopoietin-2 based on laboratory and clinical findings is shown in Figure 2.
5. Discussion
This study demonstrated that the angiopoietin-2 biomarker could be used in screening disease severity and predicting complications of leptospirosis. We found a significant relationship between angiopoietin-2 level and severe thrombocytopenia, oliguria, anemia, days of hospitalization, and CRP greater than 2+ (P-value < 0.01). Based on the findings, levels of angiopoietin-2 can predict the severity of leptospirosis at the cut point > 8.5 with AUC of 0.932, P-value < 0.0001, a sensitivity of 88.2%, a specificity of 95.7%, a positive predictive value of 91.7%, and negative predictive value of 93.8% with a positive likelihood ratio of 33.2. In-vitro studies showed that angiopoietin-2 can break the endothelial layer, and it is interesting to note that plasma exchange used in patients with severe leptospirosis decreased angiopoietin-2 levels in thrombotic microangiopathy conditions (19). According to our results, the biomarker value is accepted in the men group (P < 0.0001), people aged under 40 years (P <0.0001), and those above 40 years (P < 0.0001) with CI 95% based on the gender. On the other hand, the biomarker value is less than the acceptable range in the women group (P = 0.1396) and can be due to the low number of women (15.6%) in the present study. This difference is due to occupational and cultural conditions of subjects in the present study, and the researchers are recommended to consider it in future studies.
Consistent with the findings of the present study, Lukasz et al. reported that high levels of angiopoietin-2 were significantly associated with a complicated clinical course with the occurrence of AKI, sepsis, and intensive care unit (ICU) treatment. In their study, a total of 13 patients were involved. Disease severity criteria that were highly coordinated with high levels of angiopoietin-2 included the need for hospitalization in the ICU (P < 0.001), acute kidney injury (P < 0.0001), and CRP (P = 0.004). The relationship between CRP and angiopoietin-2 focused on the inflammatory role of angiopoietin-2 marker (19-21).
Few studies have investigated the predictive role of angiopoietin‐2 for leptospirosis. However, several studies reported the predictor value of angiopoietin‐2 for various diseases. Araújo et al. (22) reported that angiopoietin‐2 ratio is associated with severe AKI in critically ill patients as well as acute respiratory distress syndrome (ARDS). Also, Smadja et al. (23) found that angiopoietin-2 is a relevant predictive factor for ICU direct admission in COVID-19 patients. They showed that endothelial activation could reinforce the hypothesis of a COVID-19-associated microvascular dysfunction (22, 23). Moreover, Kumar et al. (24) reported that pulmonary tuberculosis is associated with elevated levels of circulating angiopoietins, possibly reflecting endothelial dysfunction. According to our findings and previous studies on the relationship angiopoietin-2 and infectious diseases, it can be used as a valuable diagnostic biomarker.
The current study had limitations, including the fact that this is a single-center study. Also, only one angiopoietin-2 measurement was obtained and followed up. Hence, we recommend measuring serum levels of angiopoietin-2 to evaluate its prognostic value in future studies.
5.1. Conclusions
Angiopoietin-2, as a biomarker, can be used in screening the disease severity and predicting the complications of leptospirosis.