1. Background
Hepatocellular carcinoma (HCC) is the fifth deadly cancer in terms of health problems. In cases where cancer leads to death, HCC comes in second place with an approximately similar incidence rate and number of deaths per year, 695900 and 748300 respectively (1, 2). However, chronic inflammation of hepatocytes seems to be the main cause of HCC progression, coexistence of inflammation and cirrhosis can also complicate diagnosis of cancer in the early stages (3). Therefore, it is necessary to find a specific biomarker for HCC that can detect cancer in a timely manner and develop prognosis predictors. These biomarkers should be able to improve the surveillance of patients.
Another factor that prevents early detection of cancer is the absence of specific clinical symptoms. In other words, in 60% of patients, cancer is detected in advanced stages with metastasis and a reduced overall survival rate (5-year < 16%). When diagnosis is performed in early stages and surgical procedures are used, the overall survival rate will improve (5-year < 93%) (4). As mentioned above, effective diagnostic markers for HCC in early stages are needed.
Multiple endocrine neoplasia (MEN) disorders are rare lesions which mostly induce tumor formation in endocrine glands (5). MEN 1 is a familial and autosomal dominant cancer related to the endocrine glands neoplasia (parathyroid glands, pituitary glands, adrenal cortical section, islet cells of the pancreas, carcinoid tumors and digestive tract) (6, 7). In MEN1 a protein called menin is encoded, with 610 amino acids, as a tumor suppressor with different expression and distribution. This protein is one of the regulators of the gene expression process. Combination of menin and ds-DNA can control the cell proliferation, cell migration, DNA damage repair, and apoptosis via a number of mechanisms (8). First of all, combination of menin and Smad3 regulates the TGF-β signaling pathway and consequently genetic transcription (9). The lack of menin increases proliferation and reduces the inhibitory effect of TGF-β and the combination between Smad3 to DNA (10). Furthermore, transforming growth factor beta can increase the production of menin in mixed-lineage leukemia cell lines (AF9). In this regard, lack of the TGF-β receptor TGF-βRII decreases the expression of menin in liver tissue of mice (11). Also, menin can affect the functions of H3K4 methyltransferases which are associated with leukemogenesis (12). Other studies have shown that there is a close relationship between menin and some other signaling proteins such as JunD proto-oncogene (JUND), peroxisome proliferator-activated receptor gamma (PPAR-γ), β-catenin, NF-κB and revealed that menin has a key role in transcription (13, 14). However, the relationship between menin protein and HCC is still unclear. Diagnosis of HCC in early stages is important. The role menin in identification of patients at risk is unknown.
2. Objectives
Hereby, we used QRT-PCR and immunohistochemistry to study the association between expression of menin and HBV-related HCC.
3. Methods
3.1. Patients
For this study, fresh specimens of liver tissue, which were prepared by needle sampling, were used. The volunteers participating in this study included 40 patients with chronic HBV alone, 41 patients with early HCC without any history of HBV, 40 patients with early HCC and co-infected with chronic HBV and 30 healthy subjects. The HCC patients were clinically at first stage, had a single tumor less than 5 cm in size. No vascular invasion was observed in these patients. Healthy individuals were selected from those who intended to donate livers and their serology markers were negative for hepatitis B, C and cancer. Also, their alanine transaminase levels were normal. Sampling was performed in two referral health centers (Shiraz and Tehran, Sep 2015 - Sep 2016). Part of the sample was rapidly transferred to -80ºC to carry out molecular assessments and gene expression as soon as possible. Another portion of the sample was fixed in neutral-buffered formalin and maintained for cellular assays (histological examination and immunohistochemistry). All operational stages of this study were performed at Zahedan University of Medical Sciences (ZAUMS) (No. 8240, IR.ZAUMS.REC.1396.63) and written consent was obtained from all participants. Diagnosis and identification of HCC patients was performed using the international criteria (15). Patients with HBV were HBsAg+ and HBV-DNA+.
3.2. Quantitative Real-Time PCR Analysis (qPCR)
After extracting total RNA from fresh tissue using the kit from a reputable company (SinaClonBioScience), according to the manufacturer’s instructions and our previous studies (16, 17), the extracted product was transferred to -80ºC for examination as soon as possible.
After evaluating the quantity and quality of the extracted RNA by electrophoresis and spectrophotometer, RNA was converted to cDNA. At this stage, a LightCycler ABI 7500 system (Applied Biosystems Inc., Foster City, CA) was used. In accordance with the instructions embedded in the 2-step RT-PCR kit (vivantis), a solution was prepared in a volume of 20 µL containing RNA, oligonucleotide (dT)-tailed primer and M-MuLV reverse transcriptase. The specific primers for menin and GAPDH (as the reference gene) were designed as follows: menin forward, 5’‑GACCCACTCACCCTCTACCA‑3’ and reverse, 5’‑GTGGTAGCCAGCCAGGTACA‑3’; GAPDH forward, 5’‑CATGAGAAGTATGACAACAGCC‑3’ and reverse, 5’‑GGGGTGCTAAGCAGTTGGTG‑3’. Annealing temperature was at 55ºC. The qPCR run was repeated twice for each sample. Finally, the results were evaluated by 2–ΔCT technique (18).
3.3. Immunohistochemical Method
After deparaffinization and rehydratation of sections, the solution of 0.3% H2O2 and autoclave with 10 mmol/L sodium citrate buffer were used to restrain the endogenous peroxidase and antigen retrieval, respectively. Monoclonal antibodies to menin (Menin Antibody B-9, SANTA CRUZ BIOTECHNOLOGY) was used for immunostainingas previously described (19, 20).
Briefly, the slides were allowed to incubate with peroxidase and protein blocker solutions at first. Then samples were reacted whit primary antibody in the presence of secondary antibody. Finally, chromogen was used to staining the slides and the intensity of biomarker expression evaluated microscopically. As positive control, sections from human tonsil tissue were used according to the manufacture’s datasheet. Negative control samples were also prepared in the same manner except that the primary antibody was not used.
The incidence level of menin was calculated depending on the intensity and extent of tissue staining in the liver samples. The coded slides were evaluated by 2 histologists. In total, 500 hepatocyte cells were examined in at least 8 - 10 fields per section. Protein expression levels were calculated using the sequential high-powered fields (×400) based on the mean number of menin+ cells. According to the staining intensity, the cells were classified in four groups; no staining or less than 5% (0), mild staining (< 25%) (1), moderate (25% - 75%) (2), and severe (> 75%) (3) (16).
3.4. Statistical Analysis
Statistical evaluations were performed by SPSS-20 software. Nonparametric Mann-Whitney test was used to analysis the intensity of menin incidence in 4 groups. Chi-square, Fisher’s exact and One-way ANOVA tests were used to compare the quantitative variables and mean levels of menin between 4 groups. P values less than 0.05 were considered as statistically significant.
4. Results
4.1. Clinicopathological Data
The demographic data and clinical information of all groups are shown in Table 1. These informations were analyzed in each group compared to the control group. Age and gender were matched in all groups and no significant difference was observed (P > 0.05). Also, there was no significant relationship between the results of immunohistochemistry and demographic data.
| Parameters | C, N (%) | HBV, N (%) | HCC, N (%) | HBV + HCC, N (%) | P | F |
|---|---|---|---|---|---|---|
| Age, y | 0.161 | 1.740 | ||||
| Mean age | 33 ± 6.216 | 53.85 ± 9.582 | 55.44 ± 10.305 | 57.13 ± 9.819 | ||
| Age range | 37 - 61 | 31 - 71 | 30 - 72 | 37 - 72 | ||
| Median | 51.50 | 58 | 56 | 59 | ||
| Sex | 0.738 | 0.413 | ||||
| Male | 24 (80.0) | 28 (70.0) | 32 (78.0) | 29 (72.5) | ||
| Female | 6 (20.0) | 12 (30.0) | 9 (22.0) | 11 (27.5) | ||
| Hepatocellular carcinoma | - | - | ||||
| Well or moderately differentiated | - | - | 37 (90.2) | 35 (87.5) | ||
| Poorly differentiated | - | - | 4 (9.8) | 5 (12.5) | ||
| HCC grading | - | - | ||||
| Early | - | - | 39 (95.1) | 38 (95.0) | ||
| G1 | - | - | 1 (2.4) | 2 (5.0) | ||
| G2-G3 | - | - | 1 (2.4) | 0 | ||
| Total bilirubin, μ mol/L | 15.43 ± 5.65 | 18.76 ± 6.75 | 28.45 ± 12.24 | 33.10 ± 11.77 | - | - |
| ALT, U/I | 26.23 ± 10.90 | 45.76 ± 32.03 | 88.25 ± 95.32 | 117.76 ± 102.54 | - | - |
| AFP, ng/mL | 2.12 ± 1.14 | 3.12 ± 2.79 | 421.21 ± 104.33 | 534.54 ± 420.76 | - | - |
| Serum HBV DNA level, mean log IU/mL (1SD) | - | 7.6 ± 0.8 | - | 7.8 ± 0.1 | - | - |
| HBs-Ag positive | - | 40 (100.0) | - | 40 (100.0) | - | - |
| HBe-Ab positive | - | 12 (30.0) | - | 15 (37.5) | - | - |
Demographic and Clinical Data of Control (C), HBV Infected (HBV), Hepatocellular Carcinoma (HCC) and HBV-Related HCC (HBV + HCC) Groups
4.2. Analysis of Relative Menin Gene Expression
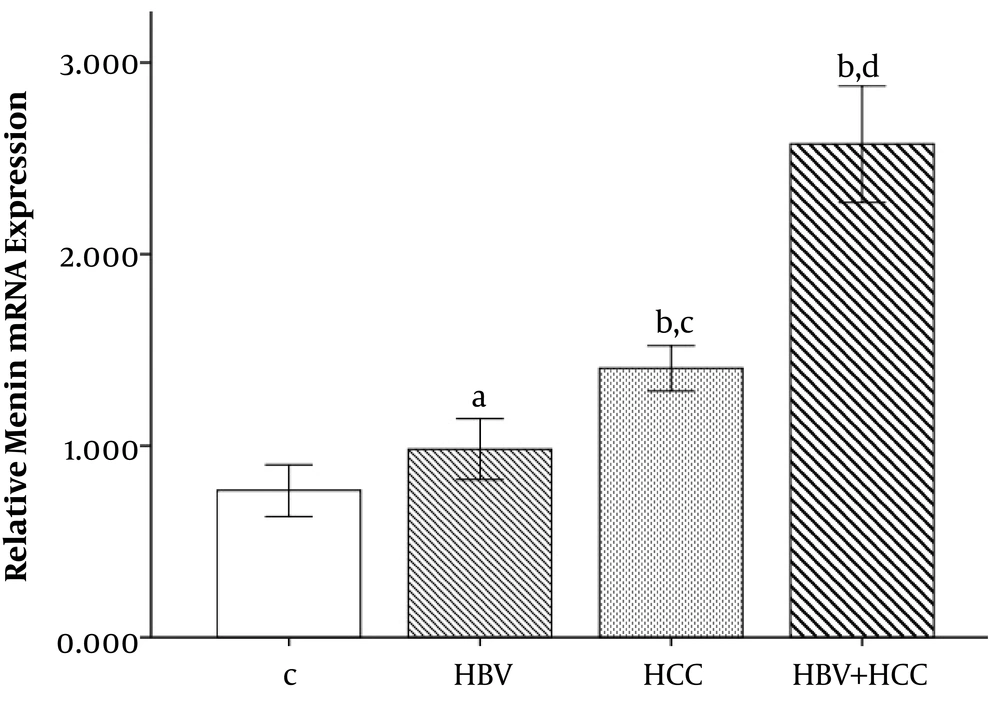
The expression levels of menin gene was calculated using qPCR technique to determine whether menin gene expression plays a role in the development of HCC in patients with chronic hepatitis B infection. Results showed a statistically significant difference between patients (HCC, HBV + HCC) and control groups in regard to the menin gene expression (Figure 1). Patients with HCC and HBV + HCC had higher levels of menin gene expression than healthy control groups (1.40 ± 0.37 and 2.57 ± 0.95 vs. 0.76 ± 0.36, respectively, P < 0.001). Moreover, HBV + HCC patients had significantly increased levels of menin gene expression than HBV patients (2.57 ± 0.95 vs. 0.98 ± 0.49, P = 0.011). Also, HBV + HCC patient had higher menin gene expression compared to the HCC and HBV groups (P < 0.001). Menin gene expression in HBV group was 0.98 ± 0.49 and was not significantly different from C group (P = 0.459).
The menin mRNA expression levels significantly increased in HBV + HCC liver tissue samples compared to controls (#P value < 0.001) and HCC cases (*P value < 0.001). Bonferroni correction PBC < 0.001. aP = 0.459 compared to control group. bP < 0.001 compared to control group, Bonferroni correction PBC < 0.001. cP = 0.011 compared to HBV group, Bonferroni correction PBC = 0.013. dP < 0.001 compared to HBV and HCC groups, Bonferroni correction PBC < 0.001.
4.3. The Menin Immunohistochemical Analysis
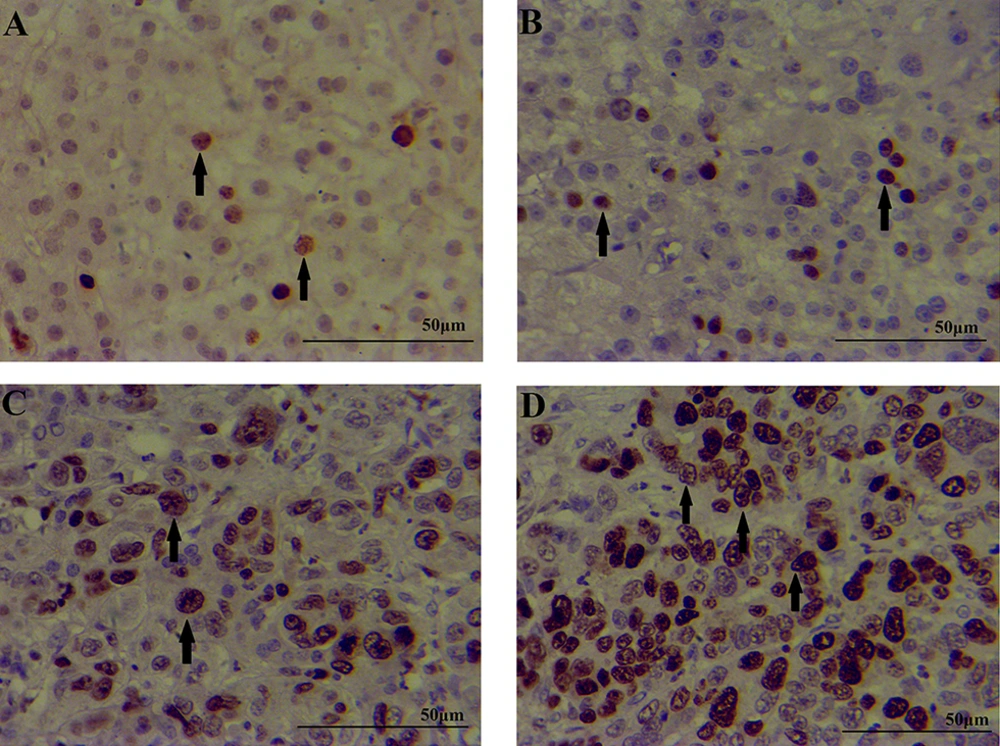
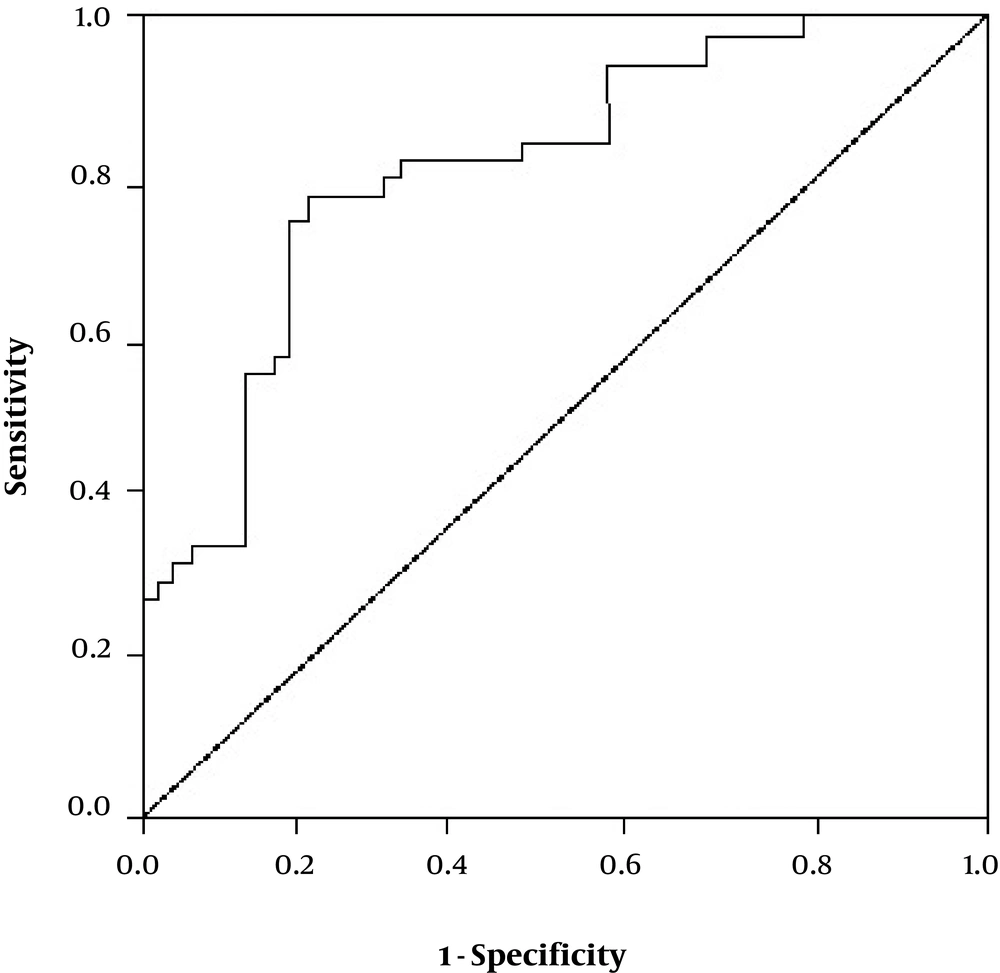
The expression of the menin protein was compared between the four groups; patients with chronic HBV infection, patients with HCC, patients with HBV + HCC and healthy controls (Table 2). The results of IHC showed that menin protein was mainly expressed in the nucleus of hepatocytes. Liver tissue of HBV+HCC patients had significantly higher levels of menin protein than patients with only HBV (P < 0.001) and also healthy controls (P < 0.001). Control group had a limited number of menin positive cells with the mean expression level of 1.68 ± 0.63 as shown in Table 2. Moreover, HBV + HCC patients had a significantly higher number of menin+ cells than HCC patients (P = 0.009). Also HCC patients had increased levels of menin protein compared to the only HBV patients (P < 0.001) and healthy subjects (P < 0.001). Menin expression in HBV group was not significantly different from C group (P = 0.225). Figure 2 showed the immunohistochemical staining patterns of menin in patients and healthy controls. The HBV + HCC patients had significantly higher menin expressions than patients with only HBV infection and/or only HCC (P < 0.001). When menin was positive, the sensitivity and the specificity were 81.5%, 77.4% respectively (Figure 3). These findings showed that menin can help us to achieve an accurate diagnosis of HCC in an early stage.
Comparing the Expression Level of Menin Liver Tissue Samples of HBV-Related HCC, HBV Infected, HCC and Healthy Control Groups
5. Discussion
Epidemiological findings have revealed a significant correlation between chronic inflammation and the development of cancer. In other words, infectious disease and persistent inflammation are responsible for cancer-related deaths because these pathological conditions can affect some signal pathways (2). It was reported that the expression level of apoptotic proteins plays important roles in HCC susceptibility, and introduction of potential HCC-related biomarkers is essential for the early detection of HCC (16). Menin, as one of the key regulatory proteins in many biological pathways and a tumor suppressor can suppress many of the vital and destructive activities of tumor cells (21).
In the current study, we focused on the relationship between menin expression and risk of HCC in patients with chronic HBV infection and revealed that the level of mRNA and protein expressions of menin in liver tissue was significantly higher in HBV + HCC patients compared to the control and only HBV groups. This study revealed that menin acts as an HCC biomarker and is overexpressed in human liver cancer.
Inactivation of the MEN1 gene can cause a rare autosomal-dominant cancer syndrome called MEN1 in a number of malignant tumors. Menin is expressed in many human tissues and cell lines. This protein is mainly found in the nucleus which means that menin perhaps regulates the transcriptional functions. There is a close relationship between menin and some transcriptional factors (22, 23) that leads to the control of cell growth and genomic integrity. Bhuiyan et al. (24) reported the upregulation of menin in tumors. They represented menin as a therapeutic target in mixed lineage leukemia-mediated leukemogenesis and introduced menin as a possible oncogenic cofactor. In this regard, we found that menin expression was higher in HCC patients compared to the other groups and associated with the existence of HBV infection. Our results show increased menin mRNA levels in patients with HBV-related HCC and indicating the involvement of menin in cancer. Menin can affect liver function through several pathways and cause cancer. Studies have shown that menin has a critical role in the TGFβ-signaling pathway which is related to the lesions of liver and HCC (22). It seems that menin has contributed to antiproliferative function of TGFβ and probably affects the functions of TGFβ. Actually, TGFβ induces hepatic cells to produce collagen I and other extracellular matrixes, and therefore it has an essential role in liver malignancies through fibrogenesis. Reports show that overexpression of menin is related to changing the extracellular matrix content in the early stages of liver lesions (25). It seems that constant and stable amounts of mRNA levels are associated with menin expression in fibrotic tissues. In this regard, Zindy et al. (26) studied the relationship between menin and collagen I by assessing the effects of menin on COL1A2 promoter activity in transfected cells. They showed that, if the expression of menin to be inhibited, COL1A2 expression is also inhibited in TGFβ- treated LX2 cells. In other words, TGFβ can induce menin expression via either regulation of COL1A2 promoter activity or TGFβ- dependent COL1A2 induction. Moreover, the studies of Smad3 and menin null mouse embryonic cells showed that menin can regulate COL1A2 expression through a TGFβ/Smad3 pathway. It means that transcriptional factors such as TGFβ probably can upregulate menin during early stages of liver malignancies (27).
Some scientists believe that menin is a scaffold protein with unknown functional motifs which cause the main function of the menin to remain inaccessible. In this regard, the study of crystal structure of menin revealed a deep pocket in menin structure which can interact with some cancer-related factors such as mixed-lineage leukemia 1 (MLL1) and JUND. In other words, menin can interact with the JUN family transcription factor JUND and/or MLL1and eventually inhibit their transcriptional activity (28).
5.1. Conclusions
Our findings clearly demonstrate an association between menin expression and the HCC risk in HBV infected patients. Our findings also emphasized that menin could be used to distinguish natural history of HCC in patients infected with HBV.
Up-regulated expression of menin was related to the pathological features of liver cancer including HBV-related HCC and may play an important role in HCC progression. Although some proteins have been introduced as prognostic biomarkers for HCC, a proper combination of menin with other biomarkers may be more useful to manage liver malignancy in different stages. Since there is a high prevalence of hepatitis worldwide, further studies are recommended to elucidate the comprehensive molecular mechanisms of menin in the oncogenesis of HCC. Since our samples were early stage hepatocellular lesions, menin may be appropriate for the diagnosis of challenging lesions in HBV patients. The applicability of the results might be limited because of the sample size. The use of larger sample size via a cohort by multiple centers could be more useful in clinical diagnosis of HBV-related HCC.