1. Introduction
Toxocariasis is a zoonotic parasitic infection with worldwide distribution, caused by Toxocara canis and Toxocara cati. The parasitic eggs are shed in the feces of definitive hosts such as dogs, cats, and other carnivores. Feline and canine feces act as a significant depot of unembryonated eggs. Eggs develop to larvae in optimal soil and environmental conditions. The transmission occurs when humans ingest embryonated eggs, usually by eating unwashed vegetables or fruits, which leads to the infection of humans with Toxocara spp. The contamination of public places with infective stages of parasitic eggs creates the risk of infection, especially for children due to their playing habits (1).
The development of second-stage larvae (L2) within eggs requires optimum temperature, adequate source of oxygen, and sufficient humidity in the environment. Temperature controls the speed of the developmental process, but as moisture in the soil prevents egg desiccation, no development will occur below the threshold levels of moisture. Environmental factors such as temperature, moisture, and soil condition can influence Toxocara development and egg survival. The strong association between climate conditions and survival of Toxocara eggs enables the development of short-term forecasting models and risk maps to estimate the risk of parasite development and disease incidence (2, 3).
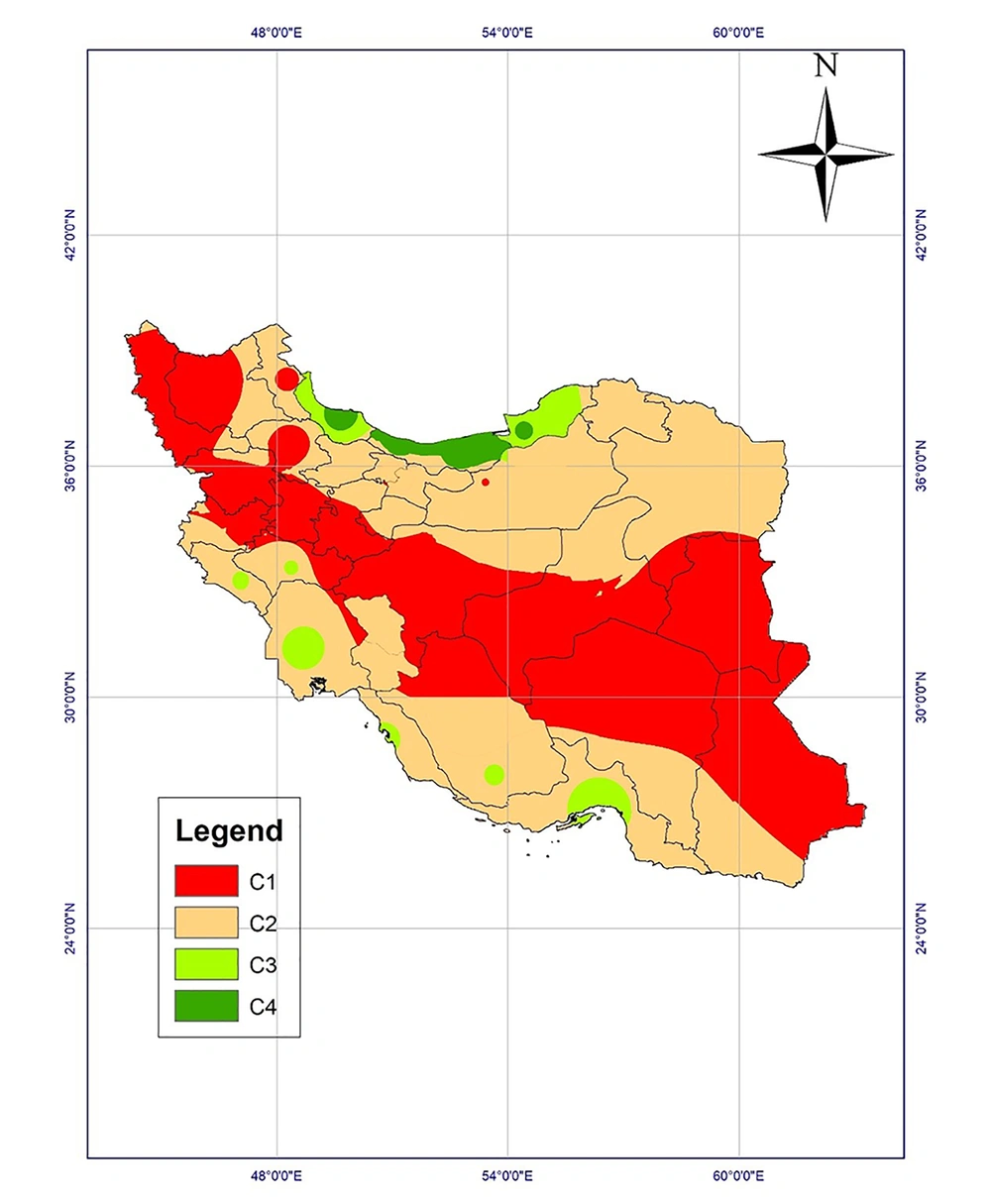
Climatic conditions can affect the maturation of Toxocara eggs based on laboratory studies. The studies examine the effect of temperature and humidity on parasitic egg development and survival to draw climate-based risk maps. Accordingly, Iran could be classified into four classes regarding the probable survival of the parasite (Table 1) (2, 4, 5).
| Class | Climatic Conditions | Frequency of Developed Eggs, % | Risk Level |
|---|---|---|---|
| C4 | Average annual temperature of 20 to 28°C, relative humidity > 50%, precipitation > 1500 mm/year | 77 | High risk |
| C3 | Average annual temperature of 15 to 20 or 28 to 32°C, relative humidity of 30% - 50%, precipitation of 700 - 1500 mm/year | 69 | Moderate risk |
| C2 | Average annual temperature of 15 to 20 or 28 to 32°C, relative humidity of 10% - 30%, precipitation of 700 - 1500 mm/year | 64 | Moderate risk |
| C1 | Average annual temperature below 10 or above 34°C, relative humidity below 10%, precipitation < 700 mm/year | < 25 | Low risk |
This study aimed to investigate the prevalence of human toxocariasis, contamination of ultimate hosts with the parasite, and soil contamination with Toxocara eggs in public places based on the previous studies and reports, as well as the classification of different provinces with regard to the survival and developmental trends of the parasitic egg in the environment.
2. Methods
Relevant studies from PubMed, Scopus, ScienceDirect, Web of Science, Google Scholar, Magiran, SID, and IranMedex databases were searched from 1 January 1955 to 30 December 2018. Data of human seroepidemiology, soil contamination with Toxocara eggs, and feline and canine toxocariasis were extracted from original studies. If there were several studies in the same area with the same subject, the highest reported prevalence would be recorded in maps. Using the data, the prevalence maps were drawn by ArcGIS 10.3 software.
The climatic data for 10 years (2007 - 2017) were obtained from Iran’s Meteorological Organization (IRIMO). The 10-year raster layers of temperature, precipitation, and relative humidity were prepared by ArcGIS 10.3 software and Kriging interpolation method. Then, a risk map of toxocariasis was drawn for the whole country.
3. Results
3.1. Spatial Distribution and Prevalence Maps
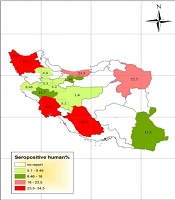
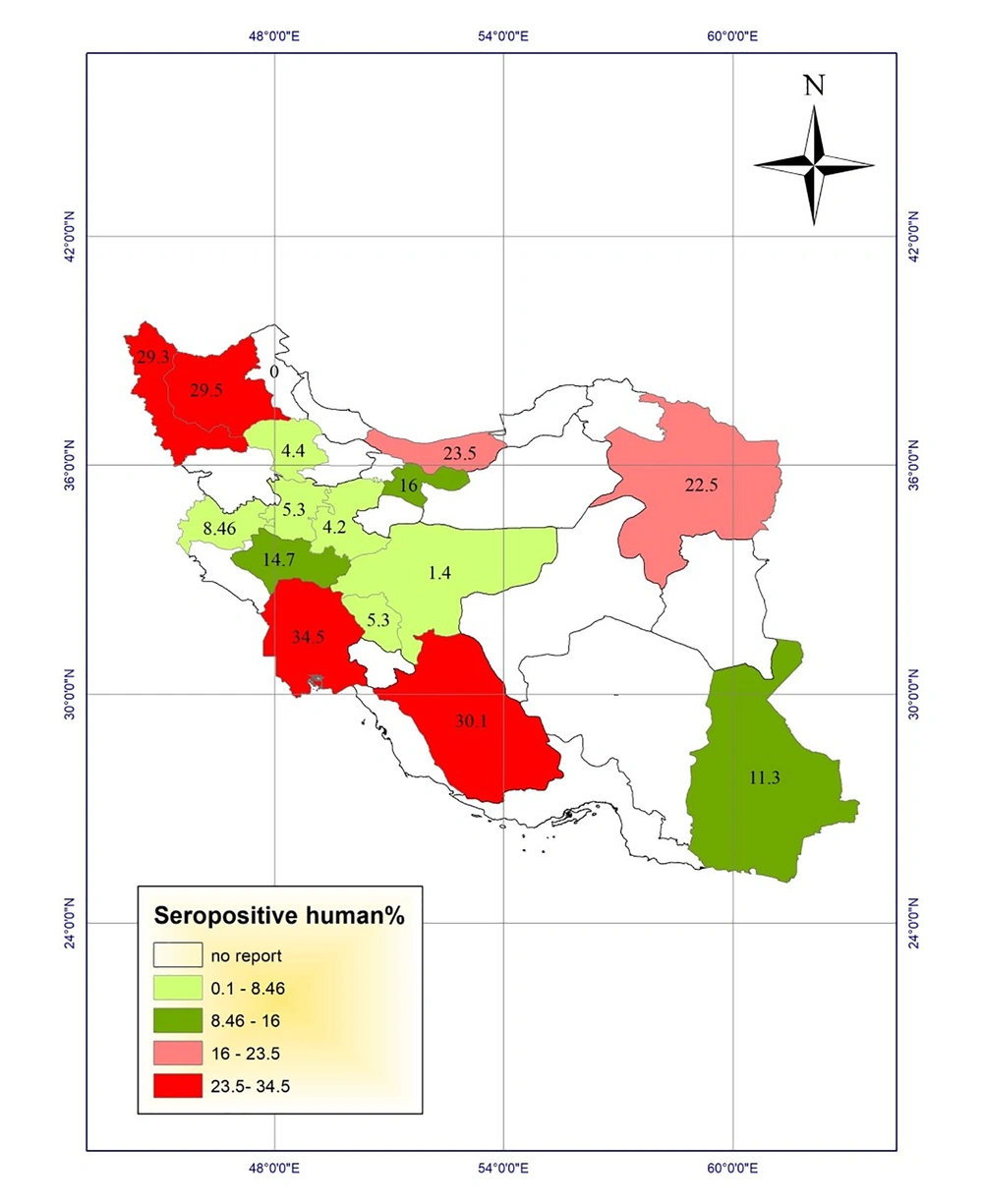
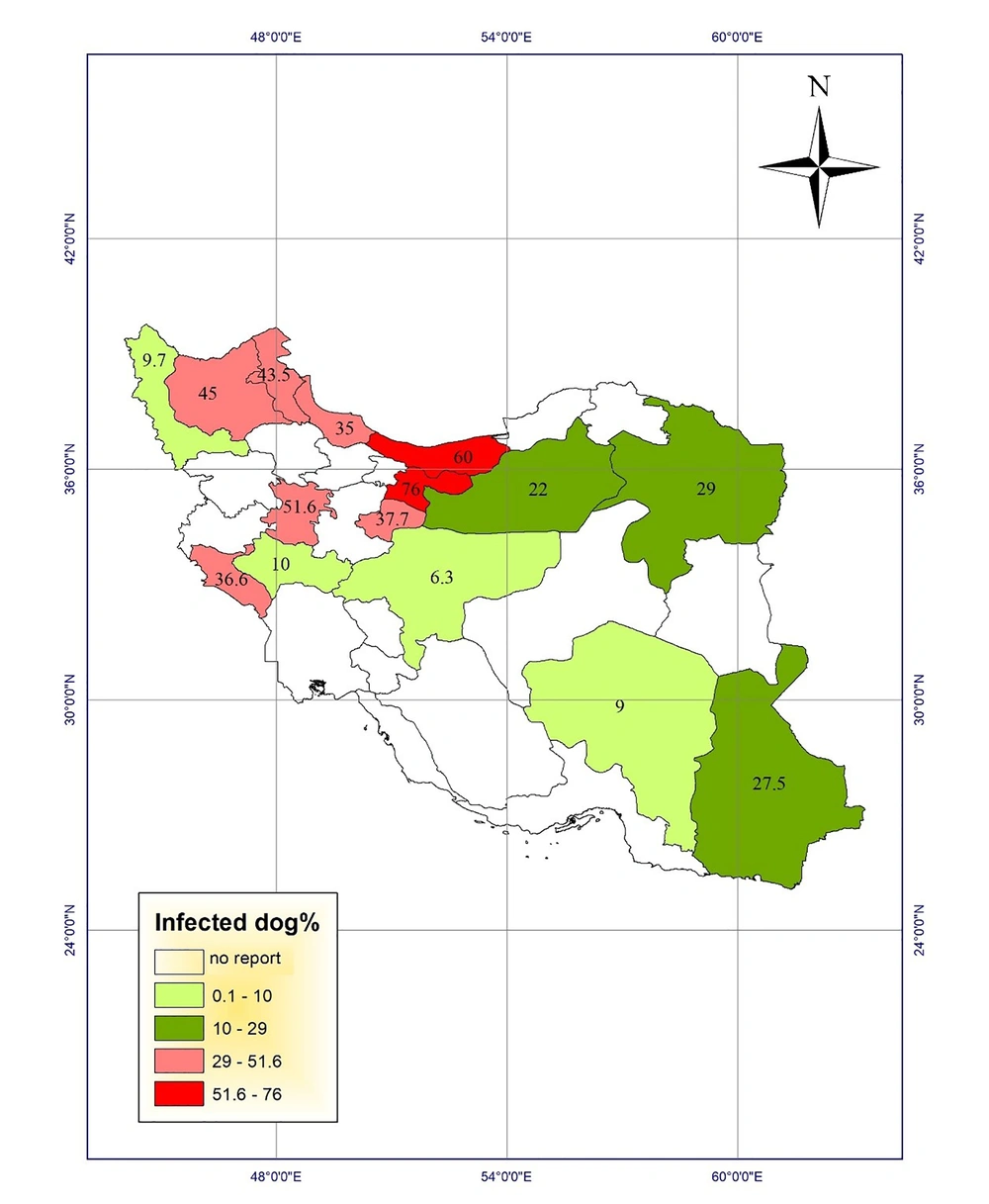
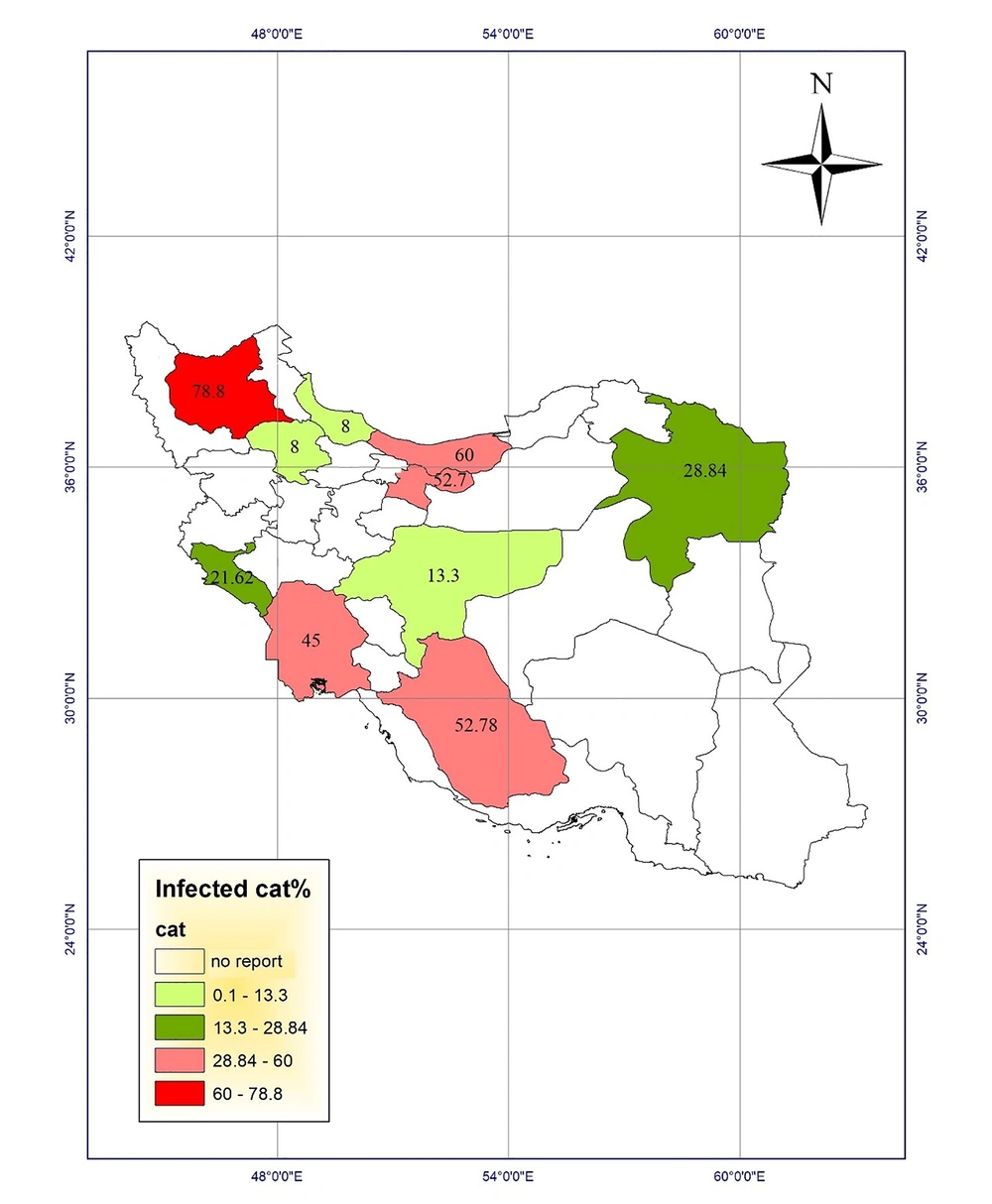
Studies of toxocariasis in Iran were mainly conducted on the contamination of carnivores. Several studies investigated the prevalence of serum toxocariasis in humans and some studies examined soil contamination in public places. Using the results, we drew the prevalence maps of disease in humans and animals and the frequency maps of soil contamination in public places of different provinces (Figures 1 - 4).
Most studies were carried out on seroepidemiology of toxocariasis in apparently healthy people with no clinical signs, especially in < 12-year-old children. The highest prevalence of human toxocariasis (34.5%) was reported in Khuzestan province among school children with chronic cough (6).
Another study in Shiraz city, Fars province, showed a prevalence rate of 30.1% for toxocariasis among 286 primary school children in urban areas of Shiraz using seropositive toxocariasis titer. In addition, the titer showed a positivity rate of 20.2% among 233 primary school children in rural areas (7, 8). On the other hand, a study conducted in Tehran on the prevalence of toxocariasis among 89 children aged 2 - 15 years reported a prevalence rate of 16% using the titer (9). Seroprevalence of toxocariasis in military men and their family in Tehran was 11.7% (10). In a study in Urmia, West Azerbaijan, of 397 serum samples, 12 (3%) were positive for anti-Toxocara IgG using ELISA. The lowest prevalence rate was reported in children aged 5 to 15 years in Isfahan province (1.4%) and asthmatic children in Arak province (1.8%) (11, 12).
Fecal contamination with Toxocara eggs in carnivores has been reported in various regions of Iran. The maximum contamination (78.8%) was reported in stray cats in East-Azerbaijan province, which were contaminated with Toxocara cati eggs (13). Another study in 1995 showed that 76% of stray dogs were infected with Toxocara canis eggs in Tehran (14). Fecal contamination with Toxocara spp. eggs in carnivores in Razavi Khorasan was reported to be 75% (15). Moreover, other studies reported the infection rates of 60% in stray dogs and cats in Mazandaran province (16). The highest rates of infection with Toxocara cati eggs in stray cats were found as 52.78% and 52.7% in Fars and Tehran provinces, respectively (7, 8, 17). In a study in Tabriz, East Azerbaijan, Khoshakhlagh et al. investigated the sensitivity of ZnSO4 flotation technique, PCR, and loop-mediated isothermal amplification (LAMP) assays for detecting Toxocara canis in feces of pet dogs. They found Toxocara canis eggs in 9%, 5%, and 11% of 100 feces of dewormed and non-dewormed dogs by microscopy, PCR, and LAMP, respectively. In addition, LAMP (10-10 to 10-13 g/µL) showed to be 10-fold more sensitive than PCR (10-10 to 10-12 g/ µL) (18).
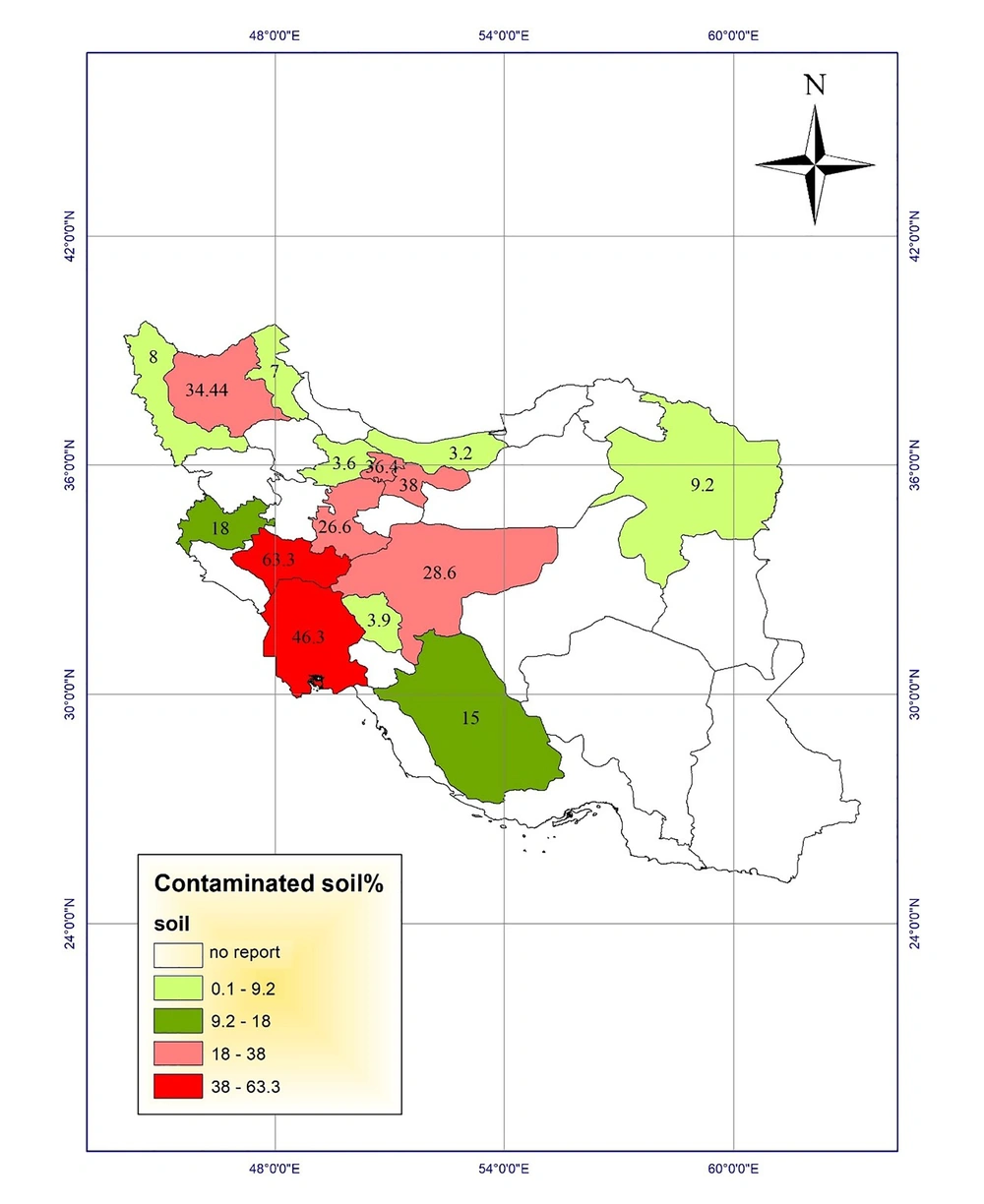
Analysis of soil contamination with Toxocara eggs in public places seems to be the most important way of studying the public health aspect of Toxocara spp. The highest soil contamination (63.3%) was reported in Lorestan province (19). Assessing soil contamination with Toxocara eggs in parks of Ahvaz city in Khuzestan showed that 46.3% of 210 samples were contaminated with Toxocara spp. eggs (20). Another study evaluated the contamination in parks in Tehran city and found that 38% of the samples were contaminated with Toxocara spp. eggs (21). In a study, Ozlati et al. pointed out the problems with identifying Toxocara spp. eggs in the soil. When the load of the parasitic eggs was low in soil samples, the microscopic method or even the conventional polymerase chain reaction (PCR) could not correctly detect the contamination. However, they found Toxocara spp. eggs in 31.6%, 7.7%, and 42.7% of 180 soil samples in Tabriz city, East Azerbaijan, using microscopy, PCR, and LAMP methods, respectively. No T. cati contamination was found by PCR while LAMP could detect the contaminations rates of 27.2%, 15.5%, and 12.2% for Toxocara cati, Toxocara canis, and mixed contamination, with a detection limit of 1 - 3 eggs/200 g soil (22).
Climate-based analysis and risk map
The climatic regions of Iran were classified into four classes and a climate-based risk map was prepared according to the possible survival and development of parasitic eggs in the environment (Figure 5). Mazandaran, Guilan, and Golestan provinces were high-risk regions. Class 3 regions, including Khuzestan, Hormozgan, Fars, Bushehr, Kohgiluyeh and Buyerahmad, Lorestan, and Ilam provinces, are the regions with proper climatic conditions, while dry and cold provinces are the regions with low-risk of Toxocara eggs survival in the environment.
4. Conclusions
Problems in the diagnosis of toxocariasis have hampered the accurate estimation of the disease prevalence in Iran. The relevant literature is limited to a few case reports and the result of seroepidemiological surveys (23). The diagnosis of Toxocara infection in humans is mainly based on para-clinical information including blood parameters, eosinophilia, IgE (immunoglobulin E) level, and serological parameters. In some cases, clinical findings can be useful; however, the clinical manifestations of the disease are usually diverse and non-specific (24).
Based on the risk map of toxocariasis, a wide area of Iran is susceptible to the presence and development of Toxocara eggs. Moreover, statistics reported by previous studies indicate that toxocariasis has a high prevalence in Iran while it is a neglected parasitic disease (6, 13, 19). Extensive areas of the country encompassing the northeast, Caspian Sea coastal, and northwest provinces, as well as western provinces along Zagros mountains and even southwestern and southern provinces provide appropriate conditions for the emergence, survival, and growth of parasitic eggs, especially Toxocara eggs, especially during wet seasons from December to April.
Based on the above-mentioned discussion, the northern and southern provinces with proper temperature and moisture are high-risk and moderate-risk regions for the survival of Toxocara eggs in the environment. Provinces in the center, southeast, and northwest of the country are regions with low risk. The effect of environmental factors on a wide range of parasitic diseases has been investigated by geographical information system (GIS), including vector-borne diseases such as leishmaniasis (25) and fascioliasis (26).
In northern provinces in hot seasons of the year, temperature, precipitation, and humidity conditions are in favor of fertilization and survival of Toxocara eggs. However, it seems that high temperature in hot seasons of the year in Southern provinces such as Khuzestan, Bushehr, and Hormozgan complicates the conditions for the survival of eggs in the environment. Further, in mountainous provinces, especially those located along Alborz and Zagros mountains, there are appropriate conditions for parasitic eggs to be embryonated, but the process in prolonged with decreased temperature.
Reports of the prevalence of toxocariasis in different provinces are in line with the above-mentioned results. The highest seroprevalence of human toxocariasis was reported in Khuzestan province (6), followed by Fars province (7, 8). Ten cases of toxocara visceral larva migrans (VLM) described in Iran were detected using IFAT (27).
The highest prevalence of contamination among carnivores was reported in East Azerbaijan (13), Tehran (14), Razavi Khorasan (15), and Mazandaran (16) provinces and the lowest prevalence of infection with Toxocara spp. among carnivores was reported from Isfahan province (11) with desert climatic conditions located in class 1 regions.
The highest soil contamination in Iran was reported in Lorestan (19), Khuzestan (20), and Tehran (21) provinces located in class 3 and 2 regions in the risk map.
Given a high prevalence of Toxocara in various parts of Iran, a high prevalence of disease in children under 10-years-old, increased contact of humans with carnivores as companion animals, and other underlying risk factors in Iran that increase the prevalence rate of toxocariasis, it is necessary to pay more attention to the disease and the treatment of final hosts of the parasite, i.e. carnivores.
The absence of a consistent program to monitor toxocariasis, whether in humans or in animals, and ignoring research priorities in high-risk regions have lead the researchers to replicate studies in a single region and ignore surveys in other areas. More attention to classification based on the risk factors of disease can be helpful in this regard. Separate studies including human, animal, and environmental studies should be conducted in different geographical areas. The outcomes will allow policymakers to set proprieties and design strategies combining accurate surveillance and prevention of this zoonotic disease.