1. Background
Of 11 million people afflicted by severe diseases who require medical attention, the burn is the 4th most common grievance of the world. In 2004, it was estimated that thermal injuries account for 300,000 deaths annually in the world (1, 2). Elderly burn patients and females are more prone to infectious complications. Furthermore, endotracheal intubation, higher percentages of burn, prolonged hospitalization, central venous access, and arterial lines, and surgical interventions all contribute to increased risk of infection in burn patients (3).
Microorganisms colonizing the burn wound originate from the patient’s endogenous flora. However, they may also be transferred to the patient through contaminated hospital environmental surfaces, water, fomites, air, and the soiled hands of health care workers (4). Following burn injury, Gram-positive bacteria, namely, Staphylococcus aureus and Enterococcus spp. primarily colonize the burn wound succeeded by Gram-negative bacteria, which its source is mainly the patient’s gastrointestinal flora (5, 6). Microorganisms transmitted from the hospital environment tend to be more resistant to antimicrobial agents compared to those originating from the patient’s normal flora (4).
Gram-negative bacteria, which constitute the predominant flora in burn patients, include Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumonia, and Enterobacter species. These organisms may often be multidrug-resistant (MDR) (7). The emergence of extended-spectrum beta-lactamase and carbapenemase-producing Gram-negative organisms, along with extensive drug-resistant (XDR) or even pan drug-resistant (PDR), is an important challenge and concern. Besides, the spread of antibiotic resistance among patients or from health personnel to patients is among the most fundamental clinical and public health problems (6, 8, 9). Development of resistance in Gram-negative bacteria towards carbapenems, which were the last-resort antibiotics, has led to failure to control the infection (10, 11). In the burn unit of Sina Educational, Research, and Treatment Center in Tabriz, which is the Reference Burn Unit for the Northwest of Iran, an escalating prevalence of antibiotic-resistant Gram-negative bacilli is observed during the last years. Despite the actions taken after diagnosis and the efforts of the health care personnel, the results were not promising, and the mortality rate seemed to get higher. Moreover, the emergence of antibiotic-resistant bacteria reduces the therapeutic options and increases the hospital stay.
This emphasizes the urgent need for standardized in-vitro susceptibility testing by clinical microbiology laboratories both for patient care and for epidemiological surveillance (4). Besides, there is a gap in studies dealing with risk factors for the infection of burn patients with resistant organisms, especially Gram-negative bacilli, and the outcome of colistin usage. Using colistin alone or with beta-lactam or aminoglycoside is almost the last resort of specialists to control most of the antibiotic-resistant infections. However, this has been a challenging task because of the inherent properties of colistin, such as its cationic nature, an affinity for plastic as well as a poor diffusibility in agar. Poor diffusion in agar negates the use of the disk diffusion technique, which is found unreliable by several studies (4, 12-15), and also of MIC testing with the gradient strip method (4, 12, 15). One of these studies have reported a good agreement between colistin gradient strip and agar dilution MIC method (4). This investigation was carried out in 1395 when agar dilution or micro-dilution techniques were not standardized. As it was important to identify the colistin susceptibility, using friendly-based techniques and methods, which can be used routinely, we compared two methods for colistin susceptibility surveillance. Moreover, risk factors associated with antibiotic-resistant organisms were also studied so that infections can be minimized, and patients' outcomes can be improved.
2. Objectives
This study aimed at to identify the risk factors associated with infections caused by Gram-negative bacilli isolated from patients hospitalized in burn units and to assess the in-vitro susceptibility of these organisms to colistin.
3. Methods
3.1. Patient Inclusion Criteria and Demographics
This descriptive-analytic study was conducted on clinical specimens of 200 burn and infected patients hospitalized in the burn unit or ICU burn ward of Sina Educational, Research, and Treatment Center, Tabriz (Iran), in 1395. Patients with clinical symptoms of infection with no history of diagnosed immunodeficiency (HIV positivity, leukemia, lymphoma, and diabetes), splenectomy, untreated chronic infection, nutrition problems, and cachexia were enrolled in the study. Cases (n = 100) were defined as patients positive for MDR or XDR or ESBL/carbapenemase producer Gram-negative bacteria in any clinical specimen cultures during the stay in burn or ICU ward. MDR was defined as acquired non-susceptibility to at least one agent in three or more antibiotic classes as described by the joint initiative on standard definitions for acquired resistance (9). A bacterial isolate was considered non-susceptible to an antimicrobial agent if was tested resistant, intermediate, or non-susceptible when using clinical breakpoints as interpretive criteria, and not epidemiological cut-offs, provided by the European Committee on Antimicrobial Susceptibility testing (EUCAST), the Clinical and Laboratory Standards Institute (CLSI) and/or the US Food and Drug Administration (FDA). MDR was defined as acquired non-susceptibility to at least one agent in three or more antibiotic classes. XDR was defined as the bacterium ‘resistant to one or more key antimicrobial agents’. Here we considered resistance to all antimicrobial classes except for colistin. Pan resistance was defined as 'resistant to all antimicrobial agents’ (9). Carbapenem resistance was defined as the resistance of a bacterium to imipenem and/or meropenem.
Controls (n = 100) were defined as patients who met the inclusion criteria but did not culture MDR or XDR or ESBL/carbapenemase producer Gram-negative bacteria during their stay in burn or ICU ward. Controls were individually matched with cases for gender and age.
To gather demographic and clinical information as well as information about risk factors such as the history of usage of antibiotics during the past two months, length of ICU stay, history of using a central venous catheter or urinary catheter, renal involvement, and mechanical ventilation, patients were interviewed.
3.2. Bacteriological Culture
Clinical specimens, including blood, wound, body fluids, endotracheal aspiration, and urine, of 200 patients admitted in burn and ICU burn wards who had clinical suspicion of infection were sent to the Division of Microbiology where bacteriological culture and identification of bacteria were performed (16). Isolation of bacterial growth was performed according to standard laboratory protocols. Briefly, bacteriological culture was performed semi-quantitatively (urine and endotracheal aspiration culture) and using the four-quadrant method (other clinical specimens) on MacConkey agar (MAC: Merck; Germany) or Eosin methylene blue agar (EMB; Merck; Germany) and blood agar (Columbia agar base; Hi-media, India). Plates were incubated for 18-24-hours, and an estimation of the relative predominance of all potential pathogens was recorded according to growth in each of the four plated quadrants. Gram-negative and/or Gram-positive isolates were identified by conventional biochemical tests as described elsewhere (16), including growth on a selective growth medium, Gram-staining, oxidase reaction, biochemical reaction in triple sugar ion agar, methyl red, motility, production of indole and hydrogen sulfide, citrate utilization test, and decarboxylation of lysine, arginine, and ornithine.
3.3. Antimicrobial Susceptibility Test
After complete biochemical identification, Gram-negative bacilli were subjected to antibiotic susceptibility test performed by the Kirby Bauer method as per CLSI protocol (16). Briefly, bacterial suspension was made from fresh bacterial culture and matched equivalent to 0.5 McFarland to spread on the Mueller-Hinton agar (MHA) plate. Antibiotic disks were then placed on the inoculated MHA plates, which were incubated for 18-24hours. Used antibiotics were as follow; ceftazidime (30 µg), cefepime (30 µg), sulfamethoxazole/trimethoprim (1.25/23.75 µg), amikacin (30 µg), gentamicin (10 µg), ceftriaxone (30 µg), ciprofloxacin (5 µg), ofloxacin (5 µg), piperacillin-tazobactam (75/10 µg), ampicillin-sulbactam (10 µg /10 µg), imipenem (10 µg), meropenem (10 µg), and colistin (10 µg) (Mast Group Co, UK). The zone of inhibition around each disk was measured and interpreted as per Clinical and Laboratory Standard Institute (CLSI) recommendations (17). Escherichia coli ATCC 25922 was used as a quality control strain. The bacteria were then classified as MDR, XDR, PDR, carbapenem-resistant depending upon the criteria described previously (9). The susceptibility of Gram-negative bacilli towards colistin was determined by disc diffusion as well as the gradient minimum inhibitory concentration (MIC) strip method. MIC ≥ 8 µg/mL was considered to be resistant, and that of ≤ 2 µg/mL was interpreted to be susceptible for P. aeruginosa, and ≥ 4 µg/mL was considered to be resistant and that of ≤ 2 µg/mL was interpreted to be susceptible for A. baumannii as per CLSI (2016) (17). As regards disc diffusion, the zone of inhibition of ≤ 11 mm was considered to be resistant, while ≥ 10 mm was taken as susceptible as per CLSI (17). For Gram-negative bacilli belonging to Enterobacteriaceae, colistin has not been suggested as per CLSI. Thus we considered the interpretative zone of P. aeruginosa for recording the susceptibility. The information obtained from the questionnaire, as well as the results obtained from the patients’ culture tests, were entered into a checklist developed by the researchers.
3.4. Statistical Analysis
Categorical variables are presented using descriptive statistics (i.e., number and percentage). Multivariable conditional logistic regression was used to assess each risk factor individually, and adjusted odds ratios (ORs) are reported. Variables with a P value < 0.25 were considered as potentially important risk factors, and those with a P value < 0.05 were considered as statistically significant. Statistical analyses were performed using IBM SPSS version 20.0 (IBM Corporation, Armonk, NY, USA).
4. Results
4.1. Patient Demographics
In the case group of patients (n = 100) who were eligible for participation, 54% were females and 46% males. In the control (n = 100) group, 48% and 52% were females and males respectively. Although it was not statistically significant, the mean age of patients in the case group was 46.54 ± 13.53 years, while in the control group, it was 48.57 ± 14.29 years.
4.2. Risk Factors
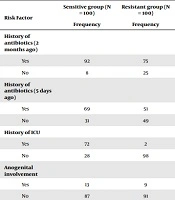
The association of potential risk factors in case and control groups are presented in Tables 1 and 2. In both groups, the most prevalent type of burn was the third-degree burn with a prevalence of 90% in the case group and 66% in the control group (P < 0.001). Eight patients in the case group had second-degree burns, among patients of the control group, 34 represented with second-degree burns. However, this difference was not statistically significant. Usage of antibiotics prior (two months) to admission had a significant (P < 0.001) association with the development of infection with antibiotic-resistant Gram-resistant organisms. Prolonged stay in the ICU Burn Unit, renal involvement, usage of the central venous catheter, urinary catheter, and the extent of the burn injury were highly significant (P < 0.001) risk factors observed in the case group compared to the control patients. The anogenital association was noticed in 13 patients of the case group, while it was observed in 9 patients of the control group. However, this association was not significant (P = 0.411). Although the mean heart and respiratory rates, as well as the body temperature in the patients of the case group, were higher than those in the control group (P < 0.001), the blood pressure was not found to be significantly different in the two groups (Table 2).
| Risk Factor | Sensitive group (N = 100) | Resistant group (N = 100) | P Value | Odd Ratio | CI Upper Limit | CI Lower Limit |
|---|---|---|---|---|---|---|
| Frequency | Frequency | |||||
| History of antibiotics (2 months ago) | 0.001 | 3.833 | 8.991 | 1.634 | ||
| Yes | 92 | 75 | ||||
| No | 8 | 25 | ||||
| History of antibiotics (5 days ago) | 0.009 | 2.139 | 3.809 | 1.201 | ||
| Yes | 69 | 51 | ||||
| No | 31 | 49 | ||||
| History of ICU | < 0.001 | 126 | 546.062 | 29.074 | ||
| Yes | 72 | 2 | ||||
| No | 28 | 98 | ||||
| Anogenital involvement | 0.366 | 1.511 | 3.713 | 0.615 | ||
| Yes | 13 | 9 | ||||
| No | 87 | 91 | ||||
| Respiratory involvement | < 0.001 | 2.266 | 2.672 | 1.922 | ||
| Yes | 21 | 0 | ||||
| No | 79 | 100 | ||||
| Central venous catheter | < 0.001 | 53.308 | 398.745 | 7.127 | ||
| Yes | 35 | 1 | ||||
| No | 65 | 99 | ||||
| Urethral catheter | < 0.001 | 81.612 | 211.763 | 31.453 | ||
| Yes | 86 | 7 | ||||
| No | 14 | 93 | ||||
| Mechanical ventilation | < 0.001 | 3.5 | 4.548 | 2.694 | ||
| Yes | 60 | 0 | ||||
| No | 40 | 100 |
Abbreviation: CI, confidence interval.
| Risk Factor | Case | Control | P Value |
|---|---|---|---|
| Age | 46.45 | 48.57 | 0.291 |
| Burning grade | 2.94 ± 0.31 | 2.66 ± 0.47 | < 0.001 |
| Burning percentage | 43.32 ± 19.22 | 6.69 ± 5.34 | < 0.001 |
| Heart rate | 107 ± 29.2 | 83 ± 14.97 | < 0.001 |
| Respiratory rate | 22 ± 7.05 | 17 ± 7.4 | < 0.001 |
| Body temperature | 38.18 ± 0.8 | 36.89 ± 0.31 | < 0.001 |
| Systolic blood pressure | 111.55 ± 13.93 | 111.91 ± 17.54 | 0.072 |
| Diastolic blood pressure | 69.82 ± 10.5 | 70.27 ± 9.84 | 0.361 |
| Wound debride (scarotomy) | 2.04 ± 1.43 | 1.64 ± 0.9 | 0.313 |
aValues are expressed as mean ± SD.
4.3. Bacterial Culture
The predominant antibiotic-resistant bacterium was Pseudomonas aeruginosa (41%), followed by Acinetobacter baumannii (25%), Klebsiella pneumoniae (10%), E. coli (6%), and Enterobacter spp. (4%). Clinical specimens send from burn patients retrieved the growth of Gram-positive and Gram-negative organisms. Details about Gram-negative organisms of each clinical specimen are presented in Table 3. P. aeruginosa and A. baumannii were the predominant organisms isolated from these patients in the two groups. According to the analysis of the antibiotic susceptibility results, 61 Gram-negative organisms were XDR, 32 isolates as MDR, and 56 isolates as carbapenem-resistant (Table 4). The results of the E-test confirmed only one Acinetobacter baumannii with MIC 256 µg/mL and, thus, resistant to colistin. Though on disk diffusion 5 Gram-negative bacteria, namely, A. baumannii (n = 1), P. aeruginosa (n = 1), Klebsiella pneumoniae (n = 1), and E. coli (n = 2) were found resistant towards colistin. However, E-test confirmed only A. baumannii. For other bacteria, the MICs were in the susceptible range. Among Gram-negative antibiotic-resistant organisms, all K. pneumoniae strains were ESBL produces while, frequency of ESBL producer E. coli and Enterobacter spp. was 83% and 50%, respectively (Table 5).
| Organisms | Wound | Blood | Urine | Endotracheal Aspiration |
|---|---|---|---|---|
| Pseudomonas aeruginosa | 10 (24) | 30 (73) | 1 (2) | 0 |
| Acinetobacter baumannii | 21 (84) | 2 (8) | 1 (4) | 1 (4) |
| Klebsiella pneumoniae | 3 (30) | 6 (60) | 1 (10) | 0 |
| E. coli | 5 (84) | 1 (16) | 0 | 0 |
| Enterobacter spp. | 3 (75) | 1 (25) | 0 | 0 |
aValues are expressed as No. (%).
| Bacteria | MDR | XDR | Pan-Resistant | Carbapenem Resistant |
|---|---|---|---|---|
| P. aeruginosa | 5 (8) | 27 (43.6) | 0 (0) | 30 (48.4) |
| A. baumannii | 7 (12.9) | 24 (44.5) | 0 (0) | 23 (42.6) |
| K. pneumoniae | 10 (47.8) | 8 (30) | 0 (0) | 3 (14.2) |
| E. coli | 7 (66) | 2 (34) | 0 (0) | 0 (0) |
| Enterobacter spp. | 3 (100) | 0 (0) | 0 (0) | 0 (0) |
| Total | 32 | 61 | 0 | 56 |
aValues are expressed as No. (%).
| Ceftazidime | Cefotaxime | Ceftriaxone | Amikacin | Gentamicin | Ciprofloxacin | Levofloxacin | Co-trimoxazole | Piperacillin/ Tazobactam | Imipenem | Meropenem | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. aeruginosa | 97 | 100 | 97 | 63 | 92 | 90 | 97 | 100 | 61 | 68 | 70 |
| A.baumannii | 100 | 100 | 100 | 92 | 100 | 98 | 100 | 92 | 98 | 98 | 98 |
| K. pneumoniae | 100 | 100 | 100 | 80 | 100 | 100 | 100 | 90 | 80 | 80 | 30 |
| E. coli | 83 | 83 | 100 | 16 | 33 | 16 | 66 | 100 | 50 | 0 | 0 |
| Enterobacter spp. | 50 | 50 | 100 | 0 | 25 | 0 | 100 | 50 | 0 | 0 | 0 |
5. Discussion
In the current study, Pseudomonas aeruginosa (41%) and Acinetobacter baumannii (25%) were the two most prevalent Gram-negative organisms causing infectious complications in burn patients. The findings are consistent with a study conducted on 3,615 patients which reported Acinetobacter baumannii (16.2%) and Pseudomonas aeruginosa (10.4%) as the most prevalent antibiotic-resistant Gram-negative bacteria involved in secondary infections in burn patients (18). Another study, which was conducted on 105 burn patients admitted in the burn unit of accidents hospital in Birmingham, reported that amongst antibiotic-resistant Gram-negative bacilli, 51% Acinetobacter anitratus, 38% Proteus spp., 29% E. coli, and 22% Pseudomonas aeruginosa were the most prevalent microorganisms (19).
Considering the lengthy stay of patients in burn units and their prolonged exposure to the hospital environment, the development of infections caused by P. aeruginosa and A. baumannii is quite expectable. The findings of the present study and other similar studies (18, 19) substantiate the fact.
According to the results of our analysis, A. baumannii had the highest level of resistance towards carbapenem, and P. aeruginosa was the most prevalent XDR, while Klebsiella pneumoniae, E. coli, and Enterobacter spp. were mainly MDR. The results of the current study are in concordance with other investigations conducted in Isfahan (Iran), whereby out of 150 Pseudomonas aeruginosa strains from burn patients, 38% were MDR and 62% XDR (20). Safaei et al. (21) investigated 96 burn patients infected with Pseudomonas aeruginosa and reported that 95.8% of the antibiotic-resistant strains were MDR, while 87.5% were XDR. In another study conducted in Belgium, De Vos et al. (22) investigated 48 burn patients infected with Acinetobacter baumannii and reported that 25% of strains were MDR.
We did not find colistin resistance among Gram-negative bacilli in the present study, except for one A. baumannii. In the study conducted by Deylam Salehi et al. (23) in Iran, the resistance to colistin among burn patients infected with Acinetobacter baumannii was reported to be 5.7%, based on the results obtained by MIC test strips. Another study, conducted in a teaching hospital in Iran, analyzed the resistance pattern of MDR Acinetobacter baumannii, and reported 100% susceptibility to colistin and 96.3% susceptibility to tigecycline (24). Newton-Foot et al. (25) investigated the National Health Laboratory Service (NHLS) database in South Africa, and after analyzing 2.6 million burn patients, they could identify 12 E. coli and 7 Klebsiella spp. resistant to colistin. Low resistance to colistin is in line with the findings of other studies (21, 23-25).
In the current study, the history using antibiotics, hospitalization in ICU, renal involvement, mechanical ventilation, and using a central venous catheter and urinary catheter were found as major risk factors for the prevalence of antibiotic-resistant infections in burn patients. Many of the participating patients of the current study had a history of using antibiotics in recent days and months. The injudicious and excessive use of antibiotics is reported as the main reason for antibiotic resistance. In the study conducted by Zilberberg et al. (26), the majority of patients who were resisted to carbapenem had a history of imprudent use of antibiotic therapy. Another research study noticed that the probability of infection with antibiotic-resistant organisms increases with an enhancement in the degree and extent of burn injuries (27), a finding which was also confirmed in our study. Zheng et al. (28) investigated the risk factors for infection with Klebsiella spp. resistant to carbapenem and observed a clear association between the history of hospitalization, especially those accompanied by carbapenem use, and the incidence of infection with antibiotic-resistant Gram-negative bacteria. They also identified invasive intubation and malnutrition as major risk factors for antibiotic-resistant infections (28). van Langeveld et al. (29) reported a significant association between the incidence of resistant infections in burn patients and factors such as ventilation for at least 21 days, prolonged use of antibiotics, and the length of hospital stay. Kumar et al. (30) investigated the risk factors for contamination with E. coli and Klebsiella spp. and found the production of extended-spectrum beta-lactamase (ESBL), usage of a urinary catheter, and history of hospitalization as the most important risk factors for infections caused by antibiotic-resistant organisms.
5.1. Conclusions
In the present investigation, a history of prior antibiotics usage, prolonged ICU stay, mechanical ventilation, and practice of catheterization were identified as the most important risk factors for the infections with antibiotic-resistant Gram-negative bacilli in burn patients. Thus, patients who are at increased risk of such infections should be monitored carefully for any development of infection, and antibiotics should be prescribed judiciously based on antibiotic susceptibility interpretations. Low resistance to colistin is beneficial, but caution should be taken, and their administration should be meticulous.