1. Background
Sepsis is a life-threatening disease worldwide with increasing incidence over the past four decades (1). Despite extensive research and improved standards of care, sepsis has remained a disorder with a high mortality rate. In the United States, sepsis develops in 970,000 people annually, more than 210,000 of whom die, and these numbers have been continuously rising (2). Sepsis is the leading cause of death in critically ill patients admitted to Intensive Care Units (ICUs) and accounts for more than 50% of in-hospital fatalities (3, 4). Sepsis mortality increases dramatically with greater disease severity: 10% - 20% for sepsis, 20% - 40% for severe sepsis, and 40% - 80% for septic shock (2)
Sepsis is accompanied by severe metabolic alterations. Thus, efforts have started and continued for many years to find some prognostic indicators among these alterations. Different biomarkers have been used for the diagnosis of sepsis, with no sufficient prognostic value. Several scoring systems such as Sequential Organ Failure assessment (SOFA) (5-8), quick SOFA (7, 9), Acute Physiology and Chronic Health evaluation (APACHE) (10-12), Simplified Acute Physiology score (SAPS) (12), and various laboratory parameters such as inflammatory cytokines (e.g., tumor necrosis factor, interleukins, C-reactive protein) (13, 14), procalcitonin (14-16), bilirubin (17), plasma lipids and lipoproteins (13, 17-23), etc., have been evaluated as prognostic factors of sepsis.
On the other hand, treatment with statins in clinical trials has led to a decrease in the severity of the disease, inflammation, and mortality of septic patients (24-28). It is believed that lower plasma levels of lipids in septic patients may decrease the ability for detoxifying endotoxins and deteriorate the patients’ conditions with effects on immunomodulation (29). However, data on serum lipid alterations in sepsis are inconclusive.
2. Objectives
Therefore, the current study aimed to investigate the lipid profile changes in patients with sepsis in a medical ICU.
3. Methods
In a prospective cross-sectional survey, we studied all adult patients who were consecutively admitted to a medical ICU of a tertiary teaching hospital for one Iranian year (21 Mar 2017 to 20 Mar 2018). The inclusion criterion was a diagnosis of sepsis by an infectious disease specialist, according to the Third International Consensus Definitions of Sepsis and Septic Shock in 2016, which defines sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection (30). The exclusion criterion was the use of any lipid-reducing medication, including statins. The demographic characteristics of patients (age, sex) and baseline serum lipid profile [triglyceride (TG), cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL)] measured in the first 24 h of admission, were recorded in a checklist for each patient. Based on mortality in the hospital, the patients were divided into two groups: survivors and non-survivors. Quantitative variables were reported as the mean (± SD). The independent samples t-test was used to compare the mean of variables between the two groups. All P values were two-sided, and P < 0.05 was considered significant for all tests. The study was conducted following the Helsinki Declaration, and it was approved by the Ethics Committee of the Deputy of Research and Technology, Urmia University of Medical Sciences, Urmia, Iran. All statistical analyses were carried out using SPSS for Windows version 16 software (SPSS Inc., Chicago, IL, USA).
4. Results
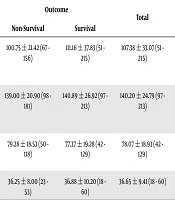
Overall, 112 patients were admitted to the ICU with a diagnosis of sepsis during the one-year study period from whom 24 patients were excluded due to the use of lipid-reducing medications. Finally, 88 patients were enrolled. After diagnostic and therapeutic care, 32 patients were eventually improved and discharged from the hospital (survival group), and 56 patients died in the hospital (non-survival group). The patients’ characteristics are summarized in Table 1. Also, Table 2 shows the lipid profiles of the two groups. The level of TG was significantly higher in non-survivors than in survivors. Cholesterol, HDL, and LDL levels did not show statistically significant differences between the two groups.
| Outcome | Total | ||
|---|---|---|---|
| Non-Survival | Survival | ||
| Mean age | |||
| Mean ± SD, y | 73.70 ± 10.43 | 67.62 ± 11.24 | 71.49 ± 11.06 |
| Range, y | (49 - 93) | (45 - 87) | (45 - 93) |
| Gender | |||
| Male | |||
| Count | 32 | 16 | 48 |
| Within gender, % | 66.7 | 33.3 | 100.0 |
| Within outcome, % | 57.1 | 50.0 | 54.5 |
| Female | |||
| Count | 24 | 16 | 40 |
| Within gender, % | 60.0 | 40.0 | 100.0 |
| Within outcome, % | 42.9 | 50.0 | 45.5 |
| Total | |||
| Count | 56 | 32 | 88 |
| Within gender, % | 63.6 | 36.4 | 100.0 |
| Within outcome, % | 100.0 | 100.0 | 100.0 |
| Indicator | Reference Value | Outcome | Total | t | P Value | |
|---|---|---|---|---|---|---|
| Non-Survival | Survival | |||||
| TG, mg/dL | Up to 150 = normal; 150 - 200 = borderline; > 200 = abnormal | 100.75 ± 21.42 (67 - 156) | 111.16 ± 37.83 (51 - 215) | 107.38 ± 33.07 (51 - 215) | 1.429 | 0.002 |
| Cholesterol, mg/dL | Up to 200 = normal; 200 - 240 = borderline; > 240 = abnormal | 139.00 ± 20.90 (98 - 181) | 140.89 ± 26.92 (97 - 213) | 140.20 ± 24.79 (97 - 213) | 0.343 | 0.112 |
| LDL, mg/dL | < 130 = normal; 130 - 160 = borderline; > 160 = abnormal | 79.28 ± 18.53 (50 - 118) | 77.37 ± 19.28 (42 - 129) | 78.07 ± 18.93 (42 - 129) | 0.452 | 0.975 |
| HDL, mg/dL | > 35 | 36.25 ± 8.00 (23 - 53) | 36.88 ± 10.20 (18 - 60) | 36.65 ± 9.41 (18 - 60) | - 0.298 | 0.20 |
5. Discussion
It is necessary to investigate the biomarkers with diagnostic and prognostic value in sepsis (18). In this study, we evaluated the lipid profiles of 88 adult patients with sepsis in a medical ICU at a tertiary teaching hospital. The mean age of the studied patients was 71.5 years, with a mortality rate of 63.6%. A recent study of the epidemiology and costs of sepsis in the United States reported the mean age of 65 years and overall mortality of 12.5%, which varied by severity (5.6%, 14.9%, and 34.2% for sepsis without organ dysfunction, severe sepsis, and septic shock, respectively] (2).
Luthold et al. (31) studied the lipid profiles of 101 critically ill adult patients who were consecutively admitted to a medical ICU in a university medical center in Basel, Switzerland. The median age was 59 (range: 23 - 86) years, and the mortality rate was 23%. Yamano et al. (17) studied 15 biochemical indices in 91 adult patients with sepsis who were on treatment for more than two weeks in the ICU of a university hospital in Osaka, Japan. The mean (± SD) age of the patients was 64 (± 18) years, with a mortality rate of 41.8%. In another study, Lee et al. (22) evaluated the lipid profile of 117 adult patients with sepsis (severe sepsis [n = 19]) and septic shock [n = 98]) who were admitted to the ICU of a large tertiary university medical center in Seoul, South Korea. The mean age of the patients was 62.7 ± 16.2 years, and the in-hospital mortality rate was 44.4%.
In another study, Tsai et al. (32) evaluated the relationship between lipid levels, inflammatory cytokines, and clinical outcomes in 103 cirrhotic adult patients with severe sepsis in the ICUs of two university hospitals in Taiwan. The mean age of the studied patients was 54.6 ± 13.1 years, and the overall in-hospital mortality for the entire groups was 64.1%. We also found two related studies in Iran. First, Barati et al. (19) compared plasma lipid levels in 70 consecutively admitted septic (n = 29) and non-septic (n = 41) adult patients with a mean ± SD age of 73.6 ± 15.7 years in an ICU in Tehran, Iran. In their study, the mortality rates were 62.1% for septic and 29.3% for non-septic patients. Second, Abdollahi et al. (18) examined the lipid profile changes in 107 adult patients with severe sepsis and septic shock in comparison with 115 non-septic patients admitted to a medical ICU in Semnan, Iran. The mean ± SD age was 74.2 ± 15.2 and 68 ± 17.3 years, and in-hospital fatality was 76.6% and 46.9% for septic and non-septic patients, respectively. Gharebaghi et al. (33) studied 139 patients with Gram-negative sepsis in the ICUs of two tertiary educational hospitals in Urmia, Iran, in 2015 and reported the mean age of 68.29 ± 17.4 years and a mortality rate of 46.8% for the patients. As can be seen, the mean age of patients with sepsis in different studies is approximately similar, but the mortality rates differ depending on the setting. In developed countries, the in-hospital mortality rates of severe sepsis and septic shock are now closer to 15% to 35% (2, 34). Still, in developing countries such as Iran, the mortality rate is higher. However, the severity of the disease is also effective in mortality rates. In epidemiological studies (2, 4), all patients with sepsis are considered, but in our survey and other similar reports (18, 19, 22, 32), patients with sepsis, severe sepsis, or septic shock were enrolled.
Regarding the lipid profile changes, although we found lower mean values for TG, total cholesterol, and HDL in the non-survival group than in the survival group, only the difference in the mean TG levels was statistically significant (P = 0.002). Similarly, Lee et al. (22) reported significantly lower levels of TG and free fatty acids in non-survival patients with sepsis on the day of admission. Barati et al. (19) also reported higher initial levels of cholesterol in non-survival than in survival patients with sepsis (101.6 ± 37.5 vs. 69.4 ± 8.3 mg/dL, respectively, P < 0.001) but no significant difference was found in TG levels.
In other studies, Luthold et al. (31) found that the HDL and total cholesterol levels were lower in infectious critically ill patients than in non-infectious critically ill patients. Also, they concluded that the diagnostic accuracy of C-reactive protein (CRP) was not better than that of HDL, but the diagnostic accuracy of procalcitonin was superior to that of HDL. Abdollahi et al. (18) also reported considerably lower cholesterol levels on the first day after admission in septic than in non-septic patients. They also found a statistically significant reverse relationship between the HDL level and mortality in septic patients. Yamano et al. (17) concluded that only low total cholesterol and high total bilirubin were associated with the prognosis of severely septic patients.
Chien et al. (35) assessed the initial serum levels of lipids and lipoproteins and their correlations with the clinical outcome of 63 patients with severe sepsis in a medical ICU of a tertiary university hospital in Taiwan. They concluded that a low HDL cholesterol level on the first day of severe sepsis was significantly associated with increased mortality and adverse clinical outcomes. In another descriptive cross-sectional study, Moini et al. (23) measured the serum lipid levels (total cholesterol, LDL, and HDL) and APACHE IV score on the first and second days after ICU admission in 100 patients. They reported significant relationships between the real mortality rate and the cholesterol level of the first and second days, the LDL levels of the first and second days, and the HDL levels of the first day. A retrospective analysis of 568 septic patients and 475 non-septic patients in a university hospital in Beijing, China, revealed that the plasma cholesterol levels in patients with sepsis were significantly lower, and the levels were significantly lower in the death group than in the survival group. The authors concluded that total cholesterol might be used as a clinical indicator to assess the outcome of patients with sepsis (36).
In terms of mechanism, in normal conditions, LDL particles transport cholesterol, phospholipids, and lipid-soluble vitamins from the liver to extra-hepatic tissues while HDL particles play a significant role in the reverse transport of cholesterol from peripheral tissues (including foam cells in the arterial wall) to the liver (37). However, plasma lipids can be affected by acute illnesses (38). The exact pathophysiological mechanisms underlying the changes in plasma lipid levels in severe illnesses and sepsis have never been fully understood (39). Lipoprotein is capable of binding to endotoxins (29). A high level of endotoxin lipopolysaccharide, as occurs in severe sepsis, depresses the activity of lipoprotein lipase and impedes triglyceride disposal, resulting in marked elevations of plasma triglycerides (23). A study claimed that acute conditions (e.g., sepsis or septic shock) induce no decrease but an increase in triglyceride production in men (23). Some authors suggested a possible role for statins (24-28, 40) and phospholipid emulsion (41) in preventing or treating sepsis. However, the anti-inflammatory effects of statins may be independent of their lipid-lowering ability (42).
Finally, the nature of the changes in the lipid profile is controversial in acute infections (19), and there are many inconsistencies among different studies. A recent study of the epidemiology and costs of sepsis in the United States reported that among 2,566,689 hospitalized patients with sepsis between 2010 and 2016, 33.1% had hyperlipidemia (2). Whether the observed changes in the lipid profile of patients with sepsis, particularly between survival and non-survival groups, are significant or not deserves further investigation, especially in the form of systematic reviews and meta-analyses.
This study had some limitations that have to be pointed out. Serum lipid levels were measured once, and the trend of lipid profile changes was not clear. Another limitation of the current study was the absence of a non-septic control group. It would be better if we used a scoring system to assess the condition of patients at the time of ICU admission.
5.1. Conclusions
Our study illustrated that the low level of triglyceride is a poor prognostic factor for the mortality of patients with sepsis. This may be attributed to alterations in serum lipid metabolism during sepsis. However, the exact mechanisms have never been fully understood. Large-scale studies are required to confirm the role of lipids and lipoproteins in the outcomes of ICU patients with sepsis.