1. Background
Toxoplasmosis is a zoonotic disease with a wide range of clinical syndromes in humans caused by Toxoplasma gondii (T. gondii), a typical coccidian parasite which is an intracellular protozoan parasite (1). Humans can be infected by eating undercooked meat of infected animals, consuming food or water contaminated with cat feces, blood transfusion, organ transplantation, or transplacentally from mother to fetus which is the most serious one. Toxoplasmosis in early pregnancy is usually accompanied by severe damage to the fetus leading to abortion or congenital malformations (2).
The infective stage of T. gondii includes three stages, sporozoite, and tachyzoite which is the rapidly multiplying form, and bradyzoite which is tissue cystic form. The tachyzoite is involved in the clinical manifestations of acute toxoplasmosis, which is susceptible to the effect of host immunity and medications. Bardyzoite is less susceptible and more resistance forms of the drugs and host immune system (3).
The prevalence of seropositivity for T. gondii fluctuates from 5% - 90% depending on age, hygiene, eating habits and geographical area. The risk of infection with T. gondii is higher in warm and humid climate. Rapid intracellular proliferation of T. gondii leads to damage of the reticuloendothelial system during the acute phase of infection (4).
Host defense mechanisms against T. gondii infection involve both cellular T-helper 1 (Th-1) and humoral T-helper 2 (Th-2) responses. Specific IgG antibodies can lyse extracellular trophozoites, but activation of T-cells and natural killer cells appear to be more important in preventing disease progression (5).
Infection with T. gondii leads to activation of T-helper cells (Th-1 and Th-2) which are involved in the synthesis and release of different cytokines, Th-1 produce interleukin-1 (IL-1), interferon-gamma(IFN-γ), and tumor necrosis factor alpha (TNF-α); while Th-2 produce interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-9 (IL-9) and interleukin-13 (IL-13) (6). In addition, nitric oxide has been found to play an important role in the immune response against growth of many protozoa including T. gondii, it is produced during the acute phase of infection leading to death of the intracellular organism (7).
Endothelial dysfunction is a systemic disorder of endothelium. The endothelium is responsible for keeping vascular tone and controlling oxidative stress by releasing mediators such as nitric oxide (NO), endothelin, and prostacyclin and regulating angiotensin-II activity. Reduction of vascular endothelium in response to appropriate stimuli is the main feature of endothelial dysfunction (8).
Endothelin 1 (ET-1), also known as preproendothelin-1 (PPET1), is a potent vasoconstrictor produced by vascular endothelial cells. Endothelin 1 is one of three isoforms of human endothelin. Pre-pro-endothelin is the precursor of the peptide ET-1. Endothelial cells convert pre-pro-endothelin to pro-endothelin and subsequently to mature endothelin, which the cells release (9). The ET-1 serum level reflects endothelial dysfunction in the different chronic infections (10).
Oxidative stress, which causes the overproduction of free radicals and/or reduction of endogenous antioxidant capacity leads to severe endothelial dysfunction. Pathogenesis of T. gondii is linked with induction of oxidative stress (11).
Lipid peroxidation and oxidative stress have been regarded as the main factors responsible for the generation of free radicals which leads to platelet and leukocyte adhesion to the vascular endothelium causing vasoconstriction and augmentation of peripheral vascular resistance. Malondialdehyde (MDA), is a biomarker of lipid peroxidation and oxidative stress, and increases in chronic acquired toxoplasmosis (12, 13).
2. Objectives
The aim of the present study was to evaluate the endothelial function in patients with acquired toxoplasmosis.
3. Methods
This case-control study involved 31 patients (27 female + 4 male) with toxoplasmosis aged 19 - 47 years matched with 20 healthy subjects (14 female + 6 male). This study was done in the Department of Clinical Pharmacology and Therapeutics in Cooperation with the Department of Medical Microbiology in the College of Medicine, Al-Mustansiriyia University, Iraq-Baghdad, 2019. This clinical study was completed and permitted by the explicit controlled ethical committee in respect to the Declaration of Helsinki. Full medical history, clinical examination and stereological tests were done for all patients and healthy controls, to confirm the acquired infection in the patients, and to exclude healthy controls with asymptomatic infections.
Exclusion criteria included; psychological diseases, neurological diseases, hypothyroidism, end-stage kidney disease, hepatic dysfunction, connective tissue disorders, malignant disorders and immunodeficiency.
3.1. Assessment of Biochemical Variables
Five milliliter of venous blood was obtained from the antecubital area of each patient and healthy subject, the blood was centrifuged at 3000/rpm and stored at -20°C for further analysis.
3.2. Serological Tests
Anti-T. gondii antibody (IgG, IgM, IgA) was determined by direct antigen-antibody reaction (CTK biotech. Inc., USA). This test is a panel rapid test and depends on lateral flow chromatographic immunoassay.
3.3. Assessment the Biomarkers of Endothelial Dysfunction
Interleukin-6 (IL-6) serum level was measured by ELISA kit method (Human IL-6 Kit, high sensitive, ab46o42, Abcam, USA) which expressed as pg/mL with a sensitivity range (1.56 - 50 pg/mL). The endothelin-1 serum level was measured by ELISA kit method (endothelin-1 ELISA Kit, ab133030, Abcam, USA) which is expressed as pg/mL with sensitivity range (0.78 – 100 pg/mL). Human malondialdehyde (MDA) was measured by ELISA kit method (Colorimetric/Flurometric, ab118970, Abcam, USA).
3.4. Statistical Analysis
The data is presented as mean ± SD and unpaired student t-test was used to determine the differences. Correlation coefficient was used to estimate the significance of correlation among different variables. Data analysis was done by using SPSS (IBM SPSS Statistics for Windows version 20.0, 2014 Armonk, NY, IBM, Corp, USA). The level of significance was considered when P < 0.05.
4. Results
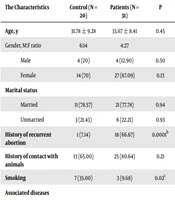
In the present study there were insignificant differences regarding age, gender, marital status and history of contact with animals (P > 0.05). On the other hand, there was significant difference concerning history of recurrent abortion, which was high in the infected patients compared with control (P = 0.0001). In addition, smoking history was low in the infected patients compared with control (P = 0.02). In this study, 100% of infected patients with T. gondii illustrated positive for IgM and 90.32% for IgG. Likewise, 15% of control subjects showed test for IgG. Furthermore, 25.80% and 74.19% of infected patients with T. gondii were treated with spiramycin and clindamycin respectively. Other characteristics of the present study are presented in Table 1.
| The Characteristics | Control (N = 20) | Patients (N = 31) | P |
|---|---|---|---|
| Age, y | 31.78 ± 9.28 | 33.67 ± 8.41 | 0.45 |
| Gender, M:F ratio | 6:14 | 4:27 | |
| Male | 4 (20) | 4 (12.90) | 0.50 |
| Female | 14 (70) | 27 (87.09) | 0.13 |
| Marital status | |||
| Married | 11 (78.57) | 21 (77.78) | 0.94 |
| Unmarried | 3 (21.43) | 6 (22.21) | 0.93 |
| History of recurrent abortion | 1 (7.14) | 18 (66.67) | 0.0001b |
| History of contact with animals | 13 (65.00) | 25 (80.64) | 0.21 |
| Smoking | 7 (35.00) | 3 (9.68) | 0.02c |
| Associated diseases | |||
| Hypertension | 9 (29.03) | ||
| Asthma | 3 (9.67) | ||
| Dyslipidemia | 8 (25.80) | ||
| Peptic ulcer | 3 (9.67) | ||
| Current therapy | |||
| Aspirin | 6 (30.00) | 14 (45.16) | |
| Theophylline | 3 (9.67) | 0.28 | |
| Statins | 8 (25.80) | ||
| Lanzoprazole | 3 (9.67) | ||
| IgM positive | 31 (100) | ||
| IgG positive | 3 (15.00) | 28 (90.32) | 0.0001b |
| Ant-toxoplasmosis agents | |||
| Spiramycin | 8 (25.80) | ||
| Clindamycin | 23 (74.19) |
aValues are expressed as mean ± SD or No. (%).
bP < 0.01.
cP < 0.05.
Regarding immunological and immunoglobulin changes in patients with acute toxoplasmosis, IgM, IgG and IgA levels were high in the infected patients compared with control (P < 0.01). Furthermore, IL-6 serum level was high in the infected patients (3.22 ± 1.61 pg/mL) compared with control (1.88 ± 0.51 pg/mL) (P < 0.01). In addition, biomarkers of endothelial dysfunction were augmented in patients with acute toxoplasmosis, ET-1 level was high in acute toxoplasmosis (7.29 ± 4.59 pg/mL) compared with control (3.11 ± 1.69 pg/mL) (P < 0.01). As well, MDA serum level was high (9.34 ± 4.17 nmol/mL) compared with control (2.87 ± 1.13 nmol/mL), (P < 0.01), Table 2.
| Variables | Control (N = 20) | Patients (N = 31) | 95% CI | P |
|---|---|---|---|---|
| IgM, g/L | 1.2 ± 0.8 | 3.6 ± 2.99 | 1.0213 - 3.778 | 0.001a |
| IgG, g/L | 4.31 ± 2.95 | 22.96 ± 9.57 | 10.1073 - 19.192 | 0.0001a |
| IgA, g/L | 1.99 ± 1.34 | 4.72 ± 2.54 | 1.4877 - 3.9723 | 0.0001a |
| IL-6, pg/mL | 1.88 ± 0.51 | 3.22 ± 1.61 | 0.5912 - 2.088 | 0.0007a |
| ET-1, pg/mL | 3.11 ± 1.69 | 7.29 ± 4.59 | 2.0230 - 6.3370 | 0.0003a |
| MDA, nmol/mL | 2.87 ± 1.13 | 9.34 ± 4.17 | 4.5462 - 8.3938 | 0.00001a |
Abbreviations: ET-1, endothelin; IL-6, interluekin-6; MDA, malondialdehyde.
aValues are expressed as mean ± SD.
aP < 0.01.
In acute toxoplasmosis, IgM serum level was significantly correlated with IgG (r = 0.55, P = 0.001), IgA (r = 0.57, P = 0.0008), IL-6 (r = 0.45, P = 0.01), ET-1(r = 0.51, P = 0.003) and MDA (r = 0.85, P = 0.0001), Table 3.
Abbreviations: ET-1, endothelin; IL-6, interluekin-6; MDA, malondialdehyde.
ar, correlation; P, significant level.
bP < 0.01.
cP < 0.05.
5. Discussion
For clinical purposes, toxoplasmosis can be divided for convenience into five infection categories, including: acquired by immune-competent patients, acquired during pregnancy, acquired congenitally; and acquired by or reactivated in immunodeficient patients, and including ocular infections. In any category, the clinical presentations are not specific for toxoplasmosis, and a wide differential diagnosis must be considered. Furthermore, methods of diagnosis and their interpretations may differ for each clinical category (14).
Toxoplasmosis in immune-competent patients is frequently asymptomatic or presented as flu-like symptoms, but in immune-compromised patients T. gondii leads to a serious fatal infection with multi-organ involvement (15). In the present study all of the recruited patients were immune-competent that explains the mild clinical presentation of acquired toxoplasmosis in the recruited patients.
Diagnosis of acute acquired infection of T. gondii depends on detection of specific antibodies against antigens of T. gondii. Serology for detection of IgM and IgG should be done in recognition of toxoplasmosis while IgA test gives supplementary evidence concerning reactivation or acute infection. An increase of IgM, IgG and IgA levels occurs in an acute infection while high IgG and IgA with negative tests for IgM occurs in reactivation but not in acute infection (16). These findings explain the high levels of IgG, IgM and IgA in the present study since all of the recruited patients were with acute infection of T. gondii. During acute toxoplasmosis, IgM appears early in plasma and rapidly declines, but IgG may persist for months to years following acute infection, therefore; a negative test for IgG excludes acute toxoplasmosis. Consequently, diagnoses of acute infection based on a fourfold increase in IgG with or without positive test for IgM as both IgG and IgM are highly sensitive and specific (17). In the present study there was a significant fivefold increase in IgG level that confirms the acute toxoplasmosis, while the IgA serum level was increased significantly compared with healthy control subjects. These findings are in agreement with Olariu et al. (18), a study that illustrated high IgA serum level is correlated with accuracy and sensitivity of the serological panel for diagnosis of acquired acute toxoplasmosis.
The results of the present study illustrated that MDA serum level was high in patients with acute toxoplasmosis compared with controls due to induction of oxidative stress and lipid peroxidation. Dincel and Atmaca’s study (19) confirmed that high level of MDA is associated with acute toxoplasmosis due to induction of oxidative stress and reduction of endogenous anti-oxidant capacity.
Indeed, the present study demonstrated a significant increase in the biomarkers of endothelial dysfunction, as both endothelin-1 and IL-6 were elevated in patients with acute toxoplasmosis compared with healthy control subjects. These findings are in concurrence with Knight et al.’ study (20), which illustrated significant endothelial damage caused T. gondii during acute infection.
It has been reported, that T. gondii induces endothelial inflammatory changes due to activation of pro-inflammatory cytokines. As well, chronic toxoplasmosis leads to cholesterol esterification, endothelial foam cell formation and development of endothelial dysfunction. Foam cells secret IL-6 which causes leukocyte adhesion and provokes the expression of adhesion molecules on the endothelial cell (21).
Moreover, free T. gondii tachyzoites cross endothelial cells via intercellular adhesion molecule-1 (ICAM-1) therefore; in vitro inhibition of ICAM-1 prevents trans-endothelial migration of T. gondii tachyzoites. In addition, monocytes and dendritic cells are highly permissive for T. gondii tachyzoites due to different susceptibility for binding to parasitic tachyzoites. These changes stimulate monocyte adhesion to the endothelium causing significant endothelial dysfunction (22).
Therefore, endothelial cells respond to these inflammatory changes by secreting IL-6 which down-regulates and attenuates endothelial damage; thus; IL-6 is regarded as a biomarker of endothelial dysfunction (23). These findings confirm our results that described high IL-6 in acute toxoplasmosis.
On the other hand, MDA is a marker of lipid peroxidation and oxidized low density lipoprotein (ox-LDL) which are increased during toxoplasmosis induced-oxidative stress. Acute oxidative stress leads to endothelial intracellular lysosomal membrane damage. Bahrami et al. (24), illustrated that T. gondii acquired cholesterol for their replication from the host LDL receptor pathway and also scavenged different lipids from the host cell.
Therefore, oxidative stress and induction of pro-inflammatory cytokines are interrelated in the induction of endothelial dysfunction in acute toxoplasmosis. Our findings revealed that levels of endothelin-1 and IL-6 were increased during acute toxoplasmosis. A recent animal model study by Estato et al. (25), showed that mice infected with T. gondii illustrated an exaggeration of leukocyte adhesion to the endothelial cells that caused endothelial dysfunction. Also, therapy with sulfadiazine improves endothelial dysfunction in acute toxoplasmosis through reduction of parasitic load and related endothelial inflammations (25).
Moreover, statins improve endothelial dysfunction in patients with toxoplasmosis due to the protective effect of statins. Besides, statins inhibit adhesion and proliferation of T. gondii (26, 27).
Moreover, toxoplasmosis was linked to the induction and progress of pregnancy induced-hypertension due to induction of oxidative stress and endothelial dysfunction. Therefore, long lasting spiramycin therapy during pregnancy reduces the risk of pregnancy induced-hypertension and pre-eclampsia (28).
5.1. Conclusions
Acute toxoplasmosis is associated with significant oxidative stress and pro-inflammatory changes which contribute in the development of endothelial dysfunction.