1. Background
It has long been known that Gram-negative bacilli (GNB) are associated with sustaining health-care associated infections (HAIs); among them, Acinetobacter baumannii (A. baumannii) is an emerging pathogen of human HAIs, with increasing morbidity and mortality worldwide (1). Hospital-acquired A. baumannii infections are typically manifested as ventilator-associated pneumonia, meningitis, bacteremia, urinary tract infections, and surgical wound infections (2).
Carbapenems have lost their efficacy against A. baumannii nosocomial infections due to increasing administration during the last years (3, 4). Growing prevalence of carbapenem-resistant and multidrug resistant A. baumannii is a major risk in care facilities. This resistance is often associated with acquisition of class D β-lactamases including OXA-type carbapenemases (OTC) such as blaOXA-58, blaOXA-24, blaOXA-143 and blaOXA-23 genes (5). Metallo β-lactamases (MBLs) and carbapenemases of Ambler class A also play important roles in this regard (6). Several pieces of evidence around the world have shown dissemination of OTC-harboring A. baumannii strains within and between hospitals (6, 7).
The two characteristics of multidrug resistance development and survival in the hospital environment have provoked A. baumannii to emerge as a successful opportunistic nosocomial pathogen (8). The hospital surface may represent as a reservoir for A. baumannii clones which easily circulate in both environmental and clinical settings. Identification of the source or reservoir of A. baumannii strains is a key factor to control future infections and ongoing outbreaks.
2. Objectives
In the current report, we evaluated the distribution of carbapenem resistant A. baumannii isolates in the environmental surfaces of five hospitals in central Iran.
3. Methods
3.1. Setting and Sampling
During a 9-month period from March to November 2018, swab samples were collected from equipment, fluids and surfaces of intensive care units (ICUs) of five hospitals (H1 to H5) in Qom City, central Iran. H1 is a gynecology hospital established in 1958, with 156 fixed beds and an active neonatal intensive care unit (NICU). H2 is the biggest university hospital in the city, established in 1982, with 500 fixed beds. H3 is a major dialysis center of the city, established in 1964, with 230 fixed beds. H4 is currently the largest trauma center of the city, established in 1945, with 276 fixed beds. Finally, H5 is established in 1990, with 158 fixed beds. Sampling was performed to reach the calculated sample size of 194 Gram negative bacilli. To this end, samples were collected from equipment and medical devices using a moistened sterile swab from a 10 cm2 surface area. Similarly, sampling of moist surfaces was carried out by rubbing the swabs on the surface area. Sampling from fluids was also performed from 1 mL of liquids.
3.2. Identification and Culture of the Isolates
The swabs were cultured on sheep blood agar and sub-cultured on MacConkey agar medium (MicroMedia, Australia). After incubation at 35°C for 48 hours, well-isolated single colonies recovered from agar plates were inoculated into triple sugar iron (TSI) agar (Merck KGaA, Darmstadt, Germany) and the results were used for discriminating nonfermenter bacteria. Acinetobacter spp. were identified based on colony characteristics, growth at 44°C, oxidase test, urease, lysine decarboxylase (MicroMedia, Australia), arginine decarboxylase, DNase, Fluorescence-Lactose-Denitrification and the oxidative fermentative test with maltose, mannitol, fructose and dextrose (Merck KGaA, Darmstadt, Germany) (9). The API 20NE system (bioMerieux, France) was used in some instances. The result of A. baumannii detection was confirmed by PCR amplification of blaOXA-51-like gene using specific primers.
3.3. Antibiotic Susceptibility Testing
The susceptibility of isolates to antibiotics was determined using the disk diffusion method according to CLSI guidelines (10). The tested antibiotics included imipenem (10 mg), meropenem (10 mg), doripenem (10 mg), cefepime, (30 mg), ceftazidime (30 mg), cefotaxime (30 mg), ceftriaxone (30 mg), amikacin (30 mg), gentamicin (10 mg), doxycycline (30 mg), minocycline (30 mg), tigecycline (15 mg), piperacillin/tazobactam (110 mg), ticarcillin/clavulanic acid (85 mg), gatifloxacin (5 mg), ciprofloxacin (5 mg), levofloxacin (5 mg), and trimethoprim/sulfamethoxazole (25 mg) (Mast Group Ltd., UK). Carbapenem-resistant A. baumannii (CRAB) was defined as resistance to at least two carbapenems, and multidrug-resistance (MDR) was defined as isolates showing non-susceptibility to at least one agent in ≥ 3 antimicrobial categories. Isolates that were non-susceptible to at least one agent in all categories except one or two antimicrobial categories regarded as extensively drug resistant (XDR) A. baumannii. The CLSI breakpoints and interpretation for zone diameter were refereed (10).
3.4. Multiplex PCR for Detection of OXA-Type Carbapenemases Genes
The standard phenol-chloroform extraction procedure was followed for genomic DNA extraction (11). A multiplex PCR was used to detect blaOXA-58, blaOXA-24, and blaOXA-23 genes in A. baumannii isolates, as described previously (12). A. baumannii reference strains NCTC 13304, NCTC 13302, and NCTC 13305 were used as positive control for blaOXA-23, blaOXA-24, and blaOXA-58 genes, respectively. The sequencing result was submitted to the GenBank database under the accession number JQ409995.1.
3.5. Enterobacterial Repetitive Intergenic Consensus-PCR
The primer pair ERIC1 (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGC-3′) were used to amplify intervening fragments of ERIC in the genomic DNA. Amplification reactions were performed in a final volume of 25 μL. Each reaction contained 2.5 μL of 10 × PCR ViBuffer, 800 μM dNTP mixture (CinnaGen, Iran), 2.5 U MaxTaq DNA polymerase enzyme (Vivantis Technologies Sdn. Bhd., Malaysia), 0.6 μM of each primer, 4000 μM MgCl2, 3 μL DNA template, and ddH2O up to 25 μL. Amplification reactions were carried out with initial denaturation (5 minutes at 95°C), followed by 35 cycles of denaturation (1 minutes at 94°C), annealing (1 minutes at 37.5°C) and extension (3 minutes at 72°C), with a final extension at 72°C for 5 minutes. Clonal relatedness of the strains was analyzed from scanned images of the agarose gel pictures using the GelCompar II software (Applied Maths, Belgium) with the band matching coefficient of Dice. The isolates were clustered using the unweighted pair-group method with arithmetic mean and displayed in dendrogram forms.
3.6. Statistical Analysis
SPSS version 22 (SPSS Inc., Chicago, IL) was used for statistical analyses of the studied parameters. The mean values between the groups were compared using an Independent sample t-test, and P < 0.05 were considered statistically significant.
4. Results
Totally, 396 swab samples were collected from surfaces and medical equipment of the five hospitals, and 1205 colonies of bacteria including 194 non-duplicate GNB were isolated (Table 1).
| Recovered Colony Number | Total Bacteria | Total GNB | Total Mean/ Sample ± SD | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H5 | ||||||||||||||
| All Bacteria | GNB | Mean/ Sample | All Bacteria | GNB | Mean/ Sample | All Bacteria | GNB | Mean/ Sample | All Bacteria | GNB | Mean/ Sample | All Bacteria | GNB | Mean/ Sample | ||||
| Equipment | 14 | 1 | 0.2 | 25 | 0 | 0 | 63 | 8 | 0.62 | 97 | 9 | 0.47 | 52 | 8 | 0.57 | 251 | 26 | 0.43 ± 0.83 |
| Dry surfaces | 47 | 10 | 0.54 | 56 | 1 | 0.05 | 178 | 7 | 0.97 | 238 | 12 | 0.85 | 162 | 11 | 0.68 | 681 | 41 | 0.51 ± 0.78 |
| Moist surfaces | 41 | 25 | 1.47 | 20 | 1 | 0.4 | 72 | 38 | 2.7 | 77 | 30 | 1.88 | 63 | 33 | 2.2 | 273 | 127 | 1.9 ± 2.37 |
| Total | 102 | 36 | 101 | 2 | 313 | 53 | 412 | 51 | 277 | 52 | 1205 | 194 | ||||||
When looking at the data of all the hospitals as a whole, the mean number of GNB colony per sample was 1.9 ± 2.37 (86.6%) in moist surfaces, which was significantly (P < 0.05) more than that in dry surfaces (0.51 ± 0.78) and medical equipment (0.43 ± 0.83). Of 151 non-fermentative bacteria, 32 isolates (21.2%) were identified as A. baumannii (five from H1, two from H2, six from H3, 12 from H4, and seven from H5). In addition, 25 strains of the genus Acinetobacter spp. other than baumannii were recorded.
The results also revealed that 17 isolates (53.1%) were CRAB, 15 were CSAB, 13 (40.6%) showed MDR phenotype, six (18.8%) showed XDR phenotype and the remaining were susceptible (Figure 1). Based on the results, six isolates showed intermediate susceptibility to tigecycline (81.3% susceptibility) and six isolates showed resistance to doxycycline (81.3% susceptibility). The rate of resistance was higher for the other antibiotics.
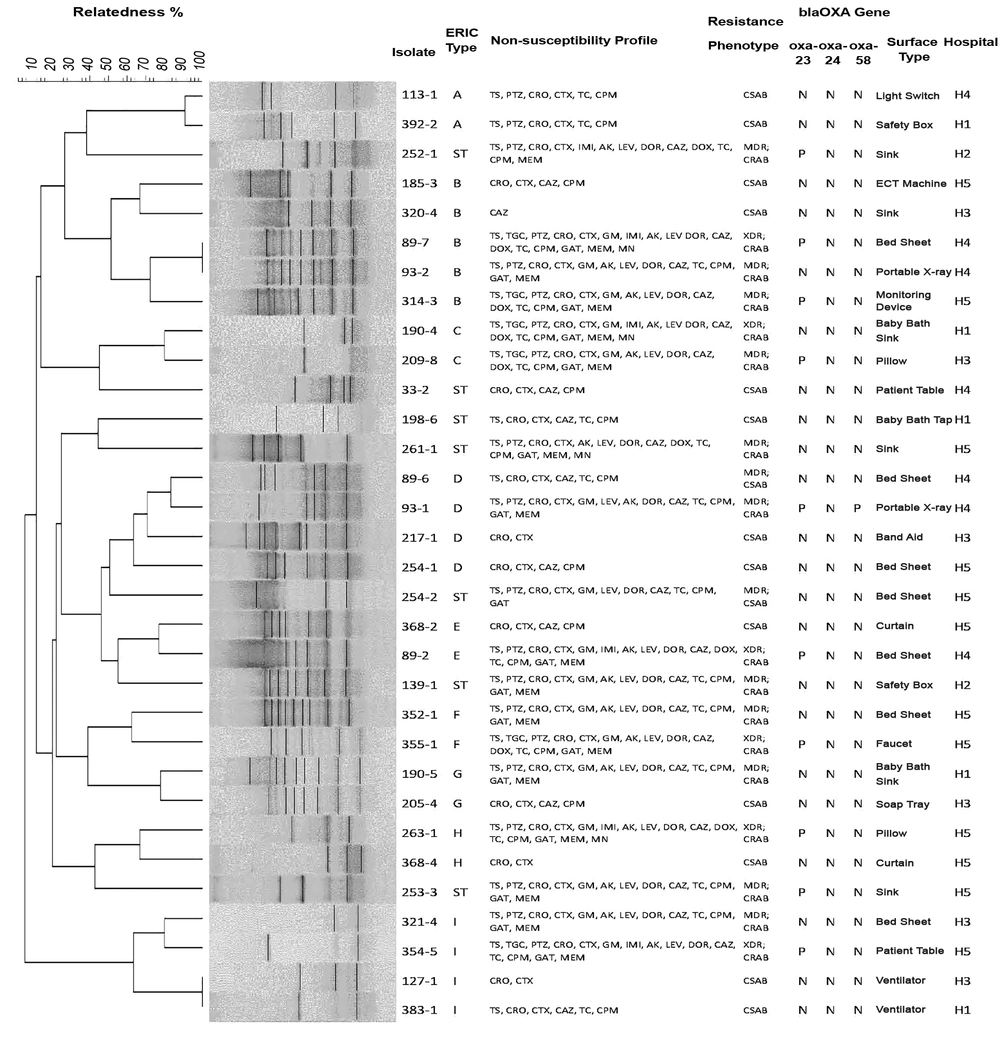
ERIC-PCR profiles and characteristics of the environmental Acinetobacter baumannii isolates. N, negative; P, positive; H, hospital; ST, single type; IMI, imipenem; MEM, meropenem; DOR, doripenem; CPM, cefepime; CAZ, ceftazidime; CTX, cefotaxime; CRO, ceftriaxone; AK, amikacin; GM, gentamicin; MN, minocycline; DOX, doxycycline; TGC, tigecycline; TS, trimethoprim-sulfamethoxazole; CO, colistin sulphate; PTZ, piperacillin-tazobactam; GAT, gatifloxacin; CIP, ciprofloxacin; LEV, levofloxacin; TC, ticarcillin-clavulanic acids.
Typing of the A. baumannii strains by ERIC-PCR provided two to 10 bands of amplification with a range of 160 to 1600 bp sizes in electrophoresis. With similarity level of at least 50%, seven single types (STs) and nine clusters (A - I) were classified. The most prevalent ERIC-PCR genotype was cluster B (15.6%). Isolates with similarity level of 100% were classified as common type (CT). In cluster B, two isolates of 89-7 and 93 - 2 in H4 were CT, which were recovered from bed sheet and portable X-ray, respectively. Moreover, in cluster I, two isolates of 127 - 1 and 383 - 1 from different hospitals (H3 and H1) were designated as CT, and were both recovered from ventilator. Further, in cluster A, two isolates of 113 - 1 and 392 - 2 from different hospitals (H4 and H1) had at least 90% similarities, and were recovered from light switch and safety box, respectively.
The multiplex PCR assay for OTC genes showed that 10 of the 32 A. baumannii isolates (31.3%) carried blaOXA-23, one isolate carried blaOXA-58, and none contained blaOXA-24. All blaOXA-23 gene positive isolates showed CRAB MDR/XDR phenotype.
Two blaOXA-23 gene positive CRAB isolates in cluster B (89 - 7, 314 - 3) were recovered from bed sheet and monitoring device in H4. From the same hospital, two other blaOXA-23 positive CRAB isolates (93 - 1 and 89 - 2) from clusters D and E were recovered from portable X-ray and bed sheet, respectively. One blaOXA-23 positive CRAB isolate was obtained from isolates of H2 (252 - 1) and H3 (209 - 8) as different genotypes. In H5, four blaOXA-23 positive CRAB isolates (253 - 3, 355 - 1, 263 - 1, and 354 - 5) with different genotypes were isolated from sink, faucet, pillow and patient table, respectively. The co-occurrence of blaOXA-58 and blaOXA-23 was found in the CRAB MDR isolate (93 - 1) of cluster D genotype from H4.
5. Discussion
There are limited reports about the investigation of A. baumannii in the hospital environment from Iran and other countries of the region (13-16). We screened surfaces and equipment of five hospitals and observed a high frequency (53.1%) of CRAB isolates which were simultaneously resistant to some tested antimicrobial agents. Previously, the high distribution of XDR A. baumannii in clinical isolates was reported in hospitals of Tehran, central Iran (7, 17). Based on the present results, tigecycline exhibited promising in vitro activity against A. baumannii isolates. This finding is in agreement with previous works, showing the highest susceptibility of A. baumannii clinical isolates to tigecyclin (18). Therefore, this antibiotic might be regarded as a treatment option for infections due to MDR/XDR A. baumannii (19, 20).
CRAB isolates were also detected on devices often used for patient care such as portable X-ray and also on surfaces touched by staff and patients. A. baumannii might first spread from infected patients or colonized personnel/visitors and then contaminate surfaces, thereby acting as prolonged sources for HAIs. Several studies suggested that A. baumannii could colonize different medical equipment and surfaces (11, 21, 22) and also could persist for years in patients (23) with the ability of biofilm formation (11). In the present study, we observed a significant MDR (21.9%) and XDR (18.8%) frequency. The XDR rate in this study was lower than the usual reported rates of clinical A. baumannii isolates, as it reached more than 90% in one report from Tehran (7). However, the MDR rate in this study was higher than reported rates of clinical isolates.
The ERIC-PCR result revealed at least four distinct clusters of A. baumannii in ICUs of all the hospitals except for H2 which showed two STs. In this study, some A. baumannii isolates with similar typing pattern were recovered from the different hospitals, indicating that clonal expansion of certain strains might take place in some of the hospitals probably through shared visitors or patients. Despite the clonal relatedness of these strains, the presence of the blaOXA-23 gene was not demonstrated for these isolates, and the resistance profiles of 127 - 1 and 383 - 1 were not identical. This discrepancy might be explained to some extent by the potential of A. baumannii to acquire foreign resistance elements as a result of selective pressure forced with antibiotics in patients and/or with antiseptics in the healthcare environment (24, 25).
A notable issue is the detection of two isolates in one clone from two geographically distinct hospitals, which were both obtained from ventilator. This suggests the possible potential of some specific genotypes of A. baumannii to resist and overcome hospital conditions, leading to inter-hospital dissemination of the same clones. Ventilator associated pneumonia due to MDR A. baumannii is one of the most common nosocomial infections, which accounts for high mortality rate in ICU hospitalized patients (26).
Detection of three different clones from baby bath sink of the H1 NICU and three different clones from bed sheet of the H4 ICU, which were all recovered in the same month, may reflect a heavy contamination source of endogenous A. baumannii and probably failure to conform to control measures and guidelines in these hospitals (27).
In different regions of Iran, OXA type β-lactamases have been widely studied in clinical isolates of A. baumannii (17, 28, 29) and it has been shown that blaOXA-23 is the most frequent OTC gene among nosocomial A. baumannii isolates (17, 30-33). However, data on the distribution of blaOXA-23 in the environmental isolates of A. baumannii is uncommon. While in this study, 31.3% of the isolates had blaOXA-23, a recent study from Iran reported that 77.5% of 40 strains isolated from air, water and surfaces carried blaOXA-23 (34). Similarly, other studies suggested higher frequency of 68.7% (15), 58% (20) and 43.3% (27) for blaOXA-23 gene positive isolates in the hospital environment, as compared to the present results. The stability and circulation of blaOXA-23 producing isolates may finally result in acquisition and horizontal transferring of genes to other strains.
We detected one blaOXA-58 positive isolate. The first known blaOXA-58 producing Acinetobacter isolate was recovered in France in 2003. The co-occurrence of blaOXA-23 and blaOXA-58 in clinical isolates has been reported occasionally (30, 35-38). There is evidence supporting the presence of blaOXA-58 carbapenemase in outbreak strains of A. baumannii and its contribution in CRAB outbreaks occurring in hospitals (38-41).
5.1. Conclusions
This study provides evidence of the presence of clonally related blaOXA-23 producing CRAB in the hospital environment, particularly on moist surfaces in ICUs of tertiary hospitals. The clinical significance of multi resistant A. baumannii is of great concern since the distribution of the same clones in different hospitals on equipment such as ventilator may increase the risk of cross-colonization of vulnerable patients. Moreover, a combination of multi-drug resistance and blaOXA-23 and/or blaOXA-58 may result in sustained surveillance of such isolates in the healthcare environment and their involvement in outbreaks.