1. Background
The parasite Blastocystis hominis was first reported by a Russian scientist in 1870; but, it was ignored due to the lack of taxonomic position (1). In 1912, it was discovered as a harmless yeast in a stool sample and officially called Blastocystis hominis. Then, it was again neglected for decades because of being harmless (2, 3). A scientist named Zierdt, recognized it as a eukaryotic unicellular organism based on morphological characteristics (4), which sparked the first light for studies in this field (5). Then, its taxonomic position was determined as a new kingdom other than that of another organism called stramenopile, which was named chromista in later revisions, based on the genetic characteristics induced by the SSUrRNA gene sequence (6, 7).
Nowadays, it is recognized as the most common intestinal parasite (8). Contrary to other intestinal pathogenic parasites, it is becoming more prevalent (9). Countries have been divided into developed (prevalence up to 10%) or developing (prevalence between 50 and 60%) groups in terms of the prevalence of this parasite (10). The main reason for its increased prevalence is the lack of health improvements (11). Infection is caused via water-resistant cysts, which are divided into two groups: Thin-walled ones that induce autoinfection inside the host and thick-walled ones that cause direct infection transfer with water and food to others (10, 12).
Even though 100 years have passed since its detection, only a few of its indications, such as morphological characteristics, have been described, and other biological indices are not well-defined; therefore, it is called a mysterious parasite (13, 14). One of the ambiguities regarding this parasite is its pathogenic characteristics. Various biological studies have recently reported the heterogeneity of this parasite and discovered its different types, which have become a basis for a few epidemiologic and pathogenic studies (8, 15-17). Furthermore, several studies have reported the effect of phenotypic, serologic, and biochemical indices on the parasites’ pathogenic characteristics (18).
Until the last decade, B. hominis had attracted attention less than what it deserved, and currently, it is receiving no attention in some countries, like Iran, mainly because of the lack of knowledge of this parasite.
2. Objectives
The aim of this study, which collected most studies in this field during recent decades, was to introduce B. hominis as a pathogen, trying to change views about this parasite and introduce it as a parasite with importance for medical sciences.
3. Methods
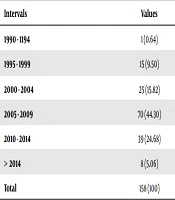
An open-ended, language-restricted (English) search was conducted in MEDLINE (PubMed), CINAHL, Scopus, and the Cochrane Library databases (from 1990 to 2018) using specific search criteria to identify Blastocystis spp. The search of the literature revealed 158 published articles on Blastocystis spp. Among these articles, the ones related to the pathogenicity of Blastocystis hominis were selected for further investigations. Table 1 presents the articles in five-year intervals, which were investigated in the present work. Table 2 shows the scope of the studied articles. Table 3 presents the target populations in the studied articles.
| Intervals | Values |
|---|---|
| 1990 - 1194 | 1 (0.64) |
| 1995 - 1999 | 15 (9.50) |
| 2000 - 2004 | 25 (15.82) |
| 2005 - 2009 | 70 (44.30) |
| 2010 - 2014 | 39 (24.68) |
| > 2014 | 8 (5.06) |
| Total | 158 (100) |
aValues are expressed as No. (%).
| Subjects | Values |
|---|---|
| Diagnostic | 49 (31.01) |
| Epidemiological | 40 (25.32) |
| Pathogenicity | 59 (37.34) |
| Others | 10 (6.33) |
| Total | 158 (100) |
aValues are expressed as No. (%).
| Study Type | Values |
|---|---|
| Symptomatic individuals | 32 (54.24) |
| Subtypes | 9 (15.25) |
| Animals | 12 (20.34) |
| Others | 6 (10.17) |
| Total | 59 (100) |
aValues are expressed as No. (%).
Studies on the pathogenicity of B. hominis have focused on humans infected with this parasite to determine the relationship between the parasite subtype and clinical signs, or laboratory sensitive animals to investigate the clinical signs and pathological and histological changes. Some articles have also focused on case studies (Table 3).
4. Results
Table 1, shows the number of articles within five-year periods. Table 2 indicates that the studies on B. hominis have mainly investigated its pathogenic characteristics, with 37.34% of the studies being focused on this aspect. In the second place are diagnostic articles that have examined the molecular methods in various countries. The epidemiologic studies are ranked third.
Based on Table 3, which presents the articles about the pathogenicity of B. hominis, various aspects of this parasite have been investigated and analyzed; in other words, a comprehensive study has been conducted on B. hominis.
5. Discussion
The present study was the first work that surveyed B. hominis from all various aspects using the collection of related articles published in 1990 - 2018.
This study showed that investigating B. hominis had an ever-increasing trend such that 60% of the studies have been carried out in the recent decade. Therefore, it can be considered an emerging parasite. Also, a great portion of studies has been conducted on the pathogenic characteristics of this parasite, which is a required field in medical science (19). This confirms the increasing attention to biological studies and their roles in the pathogenesis of the parasite. Thus, the pathogenic characteristics of B. hominis are the most important issues that have been investigated (20).
Many studies have introduced B. hominis as a potential pathogen (21-25), with digestive symptoms including diarrhea, abdominal pain, anorexia, bloat, fatigue, and extra gastrointestinal symptoms such as urticaria and itchy skin, as well as joint pain (14, 23, 26, 27). As infected people without symptoms are also found, its pathogenesis is unclear and controversial (28). Studies of the pathogenicity of B. hominis are classified into two groups based on parasite and host indices.
5.1. Parasite Indices
Some people infected with B. hominis show no clinical signs of infection, which cannot be a reason for the non-pathogenic characteristics of this parasite, because there are also other pathogenic parasites with similar conditions such as Giardia lamblia (in mild infections), Entamoeba histolytica (before E. dispar separation), and Trichomonas vaginalis (before host maturity) (29-31).
On the other hand, it is believed that the increased parasitic load can affect the pathogenicity and clinical signs and induce an acute condition for the disease (14, 32), as verified in laboratory mice models (33-36). When the scope of the studies becomes broader, the effective factors in the pathogenicity of B. hominis become more apparent; for example, by conducting genetic studies and determining the subtypes of B. hominis, it was revealed that some subtypes are pathogenic, like subtype ST1, while others are non-pathogenic, such as subtype ST2 (27, 37-39). Pathogenic subtypes vary in various geographical regions (40-44), which indicates that parasitic isolates have potentially different pathogenesis (45).
Phenotype studies have provided much information regarding the parasite. For example, all the isolates causing clinical symptoms in the host can grow in an amoeboid form in the culture medium, which is considered one of the Blastocystosis indices with clinical symptoms (46). This condition might be due to the genetic similarity of B. hominis and E. histolytica, as well as the secretion of the hyaluronidase enzyme from the B. hominis parasite that destroys proteins in the extracellular matrix and provides the condition for parasite attack. This is because this enzyme is observed at high concentrations in the urine of infected patients. This enzyme is among the pathogenic factors for E. histolytica, which destroys the base of epithelial cells (47).
Pathogenic subtypes have larger sizes and coarser surfaces and grow faster in culture medium when compared to the non-pathogenic subtypes (8). These properties can cause bacteria to have a higher tendency to attach to the parasite surface and produce more immune responses against carbohydrates attached to the bacterial cell wall such that the production of antibody IgG2 in serum and body secretions in pathogenic subtypes is 10 times the non-pathogenic cases; this indicates the immunogenic potential of pathogenic subtypes (48). Other factors like the isoenzyme model (49), the shape of proteins, and serological properties of B. hominis can also be used for separating the symptomatic cases from the asymptomatic ones; however, none of them can provide a definitive answer (18, 38, 50, 51).
Secretory IgA is considered one of the inhibiting factors; but, B. hominis secretes cysteine protease, concentrating in the vacuoles of the parasite, which decomposes secretory IgA and eliminates IgA from its path (10, 19, 52). Increased secretion of this enzyme leads to greater pathogenicity of the parasite. (35). Then, using carbohydrates such as a-D-mannosyl, a-D-glucosyl, and N-acetyl-B-D- glucosamine that are present on the cell wall of pathogenic B. hominis the parasite attaches to the epithelial cells in the intestinal mucosa and has the opportunity to multiply and colonize. (53).
When the above-mentioned glucose bind to concanavalin A (ConA) and helix pomatia agglutinin (HPA), the bound parasites are detectable by fluorescence microscopy; the same is true for the pathogenic strains of E. histolytica that produce a large quantity of lipophosphoglycan (LPG) and lipophosphopeptidoglycan (LPPG) and gain the ability to bind to epithelial cells in the intestinal mucosa; however, it is not the case of E. dispar (53). Also, it is similar to the presence of lectin in Acanthamoeba keratitis for binding to enterocytes, which are available in pathogenic forms (19).
Although it has been previously mentioned that B. hominis secretes hyaluronidase to provide the condition for the attack (54), this question is always posed whether B. hominis has the ability to attack or not. In the intestinal mucosa, there is a skeletal protein called F-action, which firmly holds the mucosa epithelial cells, increases their strength, and decreases permeability. B. hominis disturbs the distribution of this factor and consequently decreases the strength and increases permeability (35, 55, 56), which eventually results in the disturbed balance of water and electrolytes (57). In addition, B. hominis stimulates the apoptosis of host cells, which disturbs their performance. It is interesting to note that subtype 1 of B. hominis shows the highest virulence because it produces more gastrointestinal permeability (37). Increased permeability is also reported in infections with G. lamblia; but, it has not been observed in E. coli (58). Also, the secretion of cytokines IFN-γ, IL-12, and TNF-α disturbs the intestinal mucosa, as shown in the in vitro environment (53, 59).
In severe infections of mice with Blastocystis spp., the extreme infiltration of inflammatory cells, lymphocyte accumulation, mucus shedding, and parasite penetration into the intestine’s superficial layer and glandular space are reported (22, 36, 60). The increased levels of leukocytes in the stool have also been reported as one of the criteria for the level of parasite pathogenesis (23). The mechanism of diarrhea, which mostly occurs in immunocompromised patients, is not completely identified (61); however, the poison that causes diarrhea has been found in the filter of culture medium and parasite lysis (5).
The bleeding caused by enteropathy in the rectal area of a four-year-old girl, where B. hominis was the only identified agent that penetrated the superficial layer of the intestinal mucosa and glandular space accompanied by the leakage of inflammatory cells, treated by metronidazole, could verify the claim that this parasite can cause such a disorder (21).
This invasion occurs only in the large intestine because no invasion was performed during the injection of Blastocystis cyst with Entamoeba histolytica in golden hamster liver. (62).
All of the above-mentioned materials can partly indicate the role of B. hominis in the development of clinical symptoms and support its pathogenesis.
5.2. Host Indices
Hosts can also play a role in developing a disease. It has been shown that the prevalence of the parasite is higher in those with mental retardation, which can be due to the lack of sanitary considerations among this group, with more pronounced symptoms in patients with the immune system deficiency (10, 14, 63, 64). This indicates that the parasite is opportunistic; however, this claim has been rejected by some other studies (65, 66). In a study conducted on mice, it was shown that younger mice were more susceptible to infections with Blastocystis spp. (36). Also, a similar study was conducted on mice infected with Cryptosporidium, which indicated that older mice had higher resistance (67); the author discussed that the host immunity was responsible for this result.
In a study conducted on RN94-9 rats, it was shown that despite the secretion of cytokines IFN-γ, IL-12, and TNF-α in the intestinal mucosa and the increased levels of goblet cells, no significant changes were observed in colons (33), which indicated the resistance of this type of host, while it caused pathologic changes in the intestine of younger mice (36, 68).
Irritable bowel syndrome (IBS) is a digestive disorder with symptoms such as abdominal pain, diarrhea, and constipation. Inflammatory bowel disease (IBD) is also a disease associated with diarrhea and colon lesions with unknown pathogens, which have been related to infection with B. hominis in some studies (27, 69-71). In a study conducted in Europe and the Middle East, 30-40% of the patients were infected with B. hominis (72). In another study, 46% of the patients with IBS were positive for B. hominis, while only 7% of the control group had this parasite (73). It was reported that these diseases can result from serine protease, which is secreted by the parasite, and its high level can cause intense neural activity, abdominal pain, muscle cramp, and generalized pains, which are not found in bacterial and viral enteritis (74). Other studies have also demonstrated that some people infected with B. hominis have skin allergy symptoms in the form of an erythema, itching, and urticaria, where the factor is believed to be IgE secreted due to the immune system response to the parasite’s surface antigens (75-77).
6. Conclusions
Some studies strongly state that B. hominis is a pathogen, while others have ambiguities regarding this parasite, and some cases ignore its medical importance. This study aimed to collect comprehensive reasons for proving the pathogenesis of this parasite. It is hoped that further studies would fill the existing gaps regarding this parasite and identify its true identity as a medically important parasite.