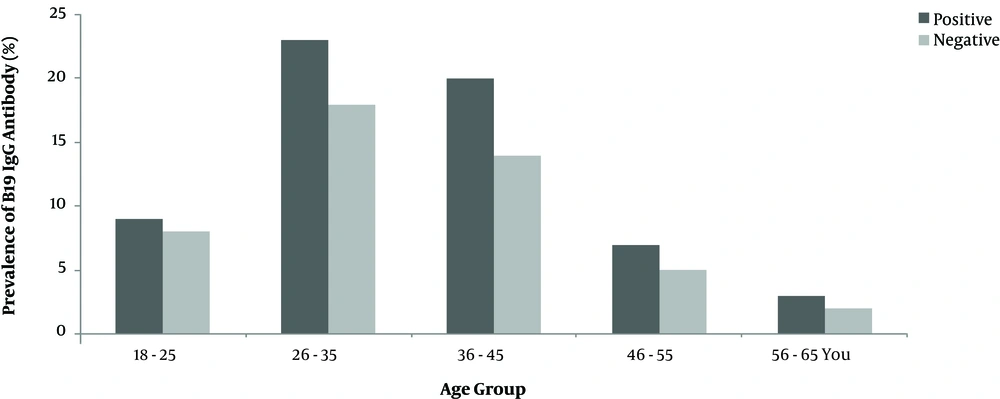1. Background
Human ParvoVirus B19 (HPV- B19) was discovered by Yvonne Cossart in 1975 (1). B19 is the only member of the Parvoviridae family known to be pathogenic in humans (2). Parvovirus B19 is a non-enveloped capsid small virus with a diameter of 22 to 24 nm. The HPV- B19 is a linear, single-strand DNA genome that encodes two structural proteins (VP1 and VP2) and a nonstructural protein NS1 (3, 4). HPV- B19 is widespread and causes a wide spectrum of clinical disorders, which varies based on the hematological and immunological status of the infected individuals. HPV- B19 only causes infection in humans, the most common of which are fifth disease, a common illness in children, chronic pure red cell aplasia, transient aplastic crisis, and prolonged joint pain in elderly individuals, hydrops fetalis, and fetal death (2, 5).
Due to the possible presence of B19 in blood or plasma products, it can be transmitted through blood transfusion, which results in severe illnesses (6). HPV- B19 viremia occurs during the first week of exposure to infection and usually lasts for 5 days, with virus titers peaking at 48 hours (7). Specific Anti B19 IgG antibodies can be detected about 15 days after infection, remain high for several months, and may persist for years (7).
Pregnant women, children, and those with chronic hemolytic anemia such as sickle cell, thalassemia, and immunocompromised patients, are at increased risk of HPV- B19 infection (8). The main transmission route of HPV- B19 is respiratory secretions, particularly when the viral load is high (9). Since the prevalence of HPV-B19 is relatively high in the general population (10), The high volume of mixed plasma samples used in the preparation of plasma-derived factors causes some contamination in blood products (11). Since HPV- B19 is a non-enveloped virus, it’s resistant to both physical and chemical inactivation techniques. Therefore, the possibility of its transmission through plasma-derived products used to treat hemophilia is relatively high (12, 13). A study on 38 serum samples from hemophilia patients reported that all of them were serologically positive for HPV- B19 infection (14). Another study that has investigated a patient who was received two lots of high-purity factor VIII concentrate reported that the patient became ill. The authors mentioned not screening the blood product for B19 –DNA using the Nucleic acid Amplification Testing (NAT) as the main probable reason (15).
2. Objectives
Few studies have investigated the transmission of HPV- B19 infection through single-donor blood components. However, some cases of anemia induced by transfusion-transmitted HPV- B19 have been reported (16-18). In this study, the seroprevalence of parvovirus B19 and the presence of B19 DNA are evaluated among blood donors in Golestan province by the ELISA and semi-nested PCR methods, respectively.
3. Methods
3.1. Samples
In this cross-sectional study, serum samples of 400 volunteer donors referred to blood transfusion centers in Golestan Province are analyzed. All samples were negative for HIV antibody (anti-HIV). Besides, the hepatitis B surface antigen (HBs Ag) and third-generation HCV antibody (anti-HCV) were selected randomly. A questionnaire was used to collect information on the age, gender, and marital status of participants. The study is approved by the local Research Ethics Committee of the High Institute for Research and Education in Transfusion Medicine.
3.2. ELISA Method
Anti-B19 (IgG and IgM) antibodies were detected by the ELISA (Enzyme-Linked Immunosorbent Assay) kit with commercial labels (Euroimmun, Germany). The ELISA methods were performed according to the instructions of the producer.
3.3. Semi-Nested PCR
Semi-nested PCR was used to detect B19 DNA. HPV- DNA extraction from serum was performed using the High Pure Nucleic Acid Extraction Kit (Roche, Mannheim, Germany). Then, the semi-nested PCR was used to detect PCR product using the following materials: 2 μL of 10 X PCR Buffer, 0.4 μL of 10mM dNTP, 1.6 μL of 25 mM MgCl2, 0.3 μL of 5 unit/μL Taq polymerase, 10.3 μL deionized water and 0.2μL of each 10 μm forward and reverse primers. At the first stage, these primers were used: NS1 (5'-ATTGCATACAGACTTTGAGC-3') as the forward primer and NS2 (5'-CAGACTTTGAGCAGGTTATG-3') as the reverse primer with 725 bp PCR product. Then 5μL of extracted DNA was added to 15 μL of PCR mixture. PCR amplification was performed for 30 cycles of denaturation for 1 min at 94º C, following by annealing for 30 seconds at 58º C, and extension for 1 min at 72º C. For the second amplification, semi-nested PCR, 2 μL of the reaction mixture was added to a new batch of 18 μL of PCR mixture containing 10 μm of each nested primers VP1 (5'-AGCATCAGGAGCTATACTTCC-3') as the forward primer and NS2 (5'-CAGACTTTGAGCAGGTTATG-3') as a reversed primer with 717 bp PCR product (19, 20). PCR products were analyzed by agarose gel electrophoresis using DNA Green Viewer (Pars tous biotechnology, Iran), as dye. A negative serum sample and B19 DNA positive serum samples were used as negative and positive controls, respectively. For negative control samples, we used a serum that was negative for all blood-borne viruses, including HBV, HCV, HIV, HTIV, HAV, and HPV- B19 with the ELISA test.
3.4. Statistical Analysis
All data were analyzed using SPSS version 23. The Chi-Square Test was used to examine the relationship between two different factors. P values less than 0.05 were considered statistically significant.
4. Results
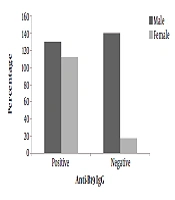
About 67% of blood donors were male, and 32.4% were female, who 67.6% of them were married. The mean age of participants was 35 ± 10 years; the youngest and oldest participants were 18 and 60 years old, respectively. Most of them (38.8%) were in the age range of 26-35 years (Table 1). Of 400 blood donors who were tested for HPV- B19 antibodies (IgM and IgG), 242 (60.5%; 95% CI) were positive and 158 (39.5%; 95% CI) were negative for B19 IgG antibody. The minimum and maximum levels of B19 IgG antibodies were 0.25 and 126.80 IU/mL, respectively. No positive case was recorded for the B19 IgM antibody.
| Variable | No. (%) |
|---|---|
| Gender | |
| Female | 130 (32.4) |
| Male | 270 (67.6) |
| Marital status | |
| Single | 92 (23) |
| Married | 308 (77) |
| Age | |
| 18 - 25 | 64 (16) |
| 26 - 35 | 156 (38.8) |
| 36 - 45 | 114 (28.7) |
| 46 - 55 | 53 (13.1) |
| 56 - 65 | 13 (3.4) |
Furthermore, no positive sample was detected for parvovirus B19 DNA by semi-nested PCR. The results of semi-nested PCR and agarose gel electrophoresis of the B19 genome are shown in Figure 1. Also, no significant association was found between age, gender, and marital status of blood donors and the seroprevalence of parvovirus B19 IgG antibody (P > 0.05) as shown in (Figure 2 and Figure 3).
5. Discussion
As a life-saving fluid, transfusion of human blood is of crucial importance to save the lives of many patients (e.g. those undergoing surgery). However, there are viruses that transmit through blood and blood products, even in cases that NAT and viral inactivation are performed. Previous studies have reported that due to the use of plasma pools containing HPV-B19 to produce batches of blood products, such as clotting factor concentrate, many of these packages are infected with HPV-B19 (21). Few studies have investigated the prevalence of B19 among blood donors in Iran. In the present study, 60.5% of samples had B19 IgG antibodies to human parvovirus B19. In a study on the sera of 730 blood donors in Tehran, the prevalence of the B19 IgG is reported as 46.3% (19). In another study performed from May-June 2016 on 500 blood donors in Tehran, the prevalence of the B19 IgG is reported as 27.6% (22) (Table 2). These differences can be attributed to factors such as the sensitivity of the test as well as seasonal and geographical variations; It worth noting that the prevalence of parvovirus B19 increases during late winter and spring.
| City | Year | Participants | No. | IgM | IgG | HPV DNA | Reference |
|---|---|---|---|---|---|---|---|
| Shiraz | 2002 | Hemophilia | 180 | ND | 74% | NDa | (23) |
| Azerbaijan | 2011 | Pregnant woman | 86 | ND | 75.6% | ND | (24) |
| Urmia | 2014 | Kidney recipients | 9 | ND | 69.2% | ND | (25) |
| Tehran | 2008 | 5 - 25 year old age | 1500 | 0.0% | 86.6% | 0.0% | (10) |
| Tehran | 2008 | Blood donor | 730 | 0.5% | 46.3% | 0.0% | (19) |
| Tehran | 2018 | Blood donor | 500 | 2.6% | 27.6% | 1.2% | (22) |
| Golestan | 2019 | Blood donor | 400 | 0.0% | 60.5% | 0.0% | Present study |
aND: No Data.
In a study conducted on blood donated by Korean donors, the prevalence of B19V DNA and anti-B19 IgG antibodies are reported as 0.0% and 60%, respectively (Table 3) (26), which is consistent with the prevalence of IgG in the present study. The prevalence of IgG anti-B19 in Chile, China, Tunisia, Italy, Spain, and India is reported as 54.8%, 55%, 43%, 79%, 64.7%, and 27.96%, respectively (21, 27-31).
There is evidence that suggests the prevalence of HPV-B19-specific-IgG antibodies is directly associated with age. A study conducted in Tehran on children aged 5 - 9 years and adults aged 21-26 years has reported a prevalence of 79.3 and 86.6% for B19 IgG, respectively (10). Increasing the seroprevalence with age means that the proportion of people at risk to the virus decreases with increasing age, and this group is immune (10). In a study on hemophilia patients in Shiraz, a high percentage of patients was positive for anti- B19 IgG compared to the control group (74% vs. 56%) (32).
In another study performed on pregnant women in West Azerbaijan in 2011, a prevalence of 75.6% is reported (23). Also, the prevalence of HPV- B19 (69.2%) is reported to be high among kidney recipients in Urmia (Iran) (24).
In this study, no IgM positive sample was found. In a study performed on sera of 730 blood donors in Tehran, the prevalence of IgM is reported as 0.5%. Doyle et al. reported a seroprevalence of 1% among American blood donors (33), whereas Munoza reported a prevalence of 0% among Spanish blood donors (31). Also, in the present study, all sera samples were negative, as determined by sensitive and specific semi-nested PCR. The sensitivity of the NAT method significantly influences the prevalence of B19 viremia in blood and plasma of donors, so that it may range from 0.003% to 0.88% (25). Low levels of HPV- B19 DNA (i.e., 10 to 103 IU/mL) with anti-HPV-B19 IgG may persist for three to five years in immunocompetent blood donors (25). Many of the samples negative for B19 DNA are positive for anti-B19 IgG antibodies, which indicates that the majority of donors have been exposed to the virus and, therefore, have developed the antibody response. Currently, most developed countries do not implement necessary measures to prevent the transmission of B19 through blood and blood products because the dominant view is that blood components with low levels of B19 DNA do not transmit B19 infection (34, 35).
In conclusion, the present study demonstrated a high IgG prevalence among donors in Golestan Province. Also, none of the samples was positive for B19 DNA, which indicates the low risk of B19 transmission through blood transfusion. The presence of anti-B19 IgG in the blood of the donors or recipients leads to neutralization of the virus, which in turn prevents the infection. Donors with persistent IgG anti- HPV- B19 might be considered as “HPV- B19 -safe" for single-donor blood components. Regardless of the high prevalence of anti-B19 IgG in Iranian blood donors, transfusion-transmitted human HPV- B19 is considered as an unresolved problem, particularly for those with high sensitivity such as pregnant women, immune-deficient patients, and patients with hematological problems. Hence, implementing preventive measures with a special focus on these groups would be useful.