1. Background
Tick-borne encephalitis (TBE) is a viral tick-borne neurological infectious disease, occurring in different areas of Eurasia, including Central, Eastern, and Northern Europe, as well as Northeastern Asia (1). The causative agent of TBE is a neurotropic positive-sense single-stranded RNA virus, belonging to the genus Flavivirus from the family Flaviviridae. TBE virus (TBEV), first detected in 1939, is historically classified into three subtypes, including European, Siberian, and Far-Eastern subtypes (2), which are endemic in Central, Eastern, and Northern Europe; Eastern Europe, Russia, and Northern Asia; and Eastern Russia, China, Korea and Japan, respectively. Recently, two other subtypes designated as the Baikalian and the Himalayan have also been identified (3).
In nature, TBEV circulates in an enzootic cycle between ticks and mammals. Small mammals such as rodents and insectivores are incriminated as natural reservoir hosts (4). Humans may incidentally acquire infection via tick bites or consumption of raw and unpasteurized dairy products (5). Ticks of the genus Ixodes (I. ricinus for the European subtype and I. persulcatus for the Siberian and the Far-Eastern subtypes) are the main vectors of the virus (5). The majority of infected cases are asymptomatic. In symptomatic cases, the disease occurs following an incubation period of two to ten days. Most patients experience a typical biphasic disease, involving the first stage of the disease (five days on average), one week of remission, followed by the second stage of the disease.
The first stage of TBE is characterized by influenza-like symptoms, such as fever, headache, myalgia, fatigue, and vomiting (6). In the second stage of TBE, patients experience neurological disorders, ranging from mild meningitis to severe encephalitis.
The fact that the geographical distribution of TBE corresponds to the distribution of its vectors is no secret (7). In fact, due to global warming, the geographical distribution and incidence of this disease have increased in recent years (8-11). Annually, around 15000 cases of TBE are reported in Europe and Asia (1, 12). With the increasing tourism industry, TBE has become a more global problem.
Effective vaccines are available against TBE and vaccination is highly recommended for high-risk groups living in endemic countries (13). Additionally, measures to avoid tick bites such as using tick repellents and wearing long clothes are also recommended (14).
Occurrence of the main TBEV vector, I. ricinus, has been documented in Northern Iran (15), especially in the Mazandaran Province (16, 17). However, there is no information about the circulation of TBEV in Iran.
2. Objectives
Therefore, the present study was aimed to investigate the serological profile of TBE to evaluate its seroepidemiological status for the first time in Mazandaran Province in Northern Iran.
3. Methods
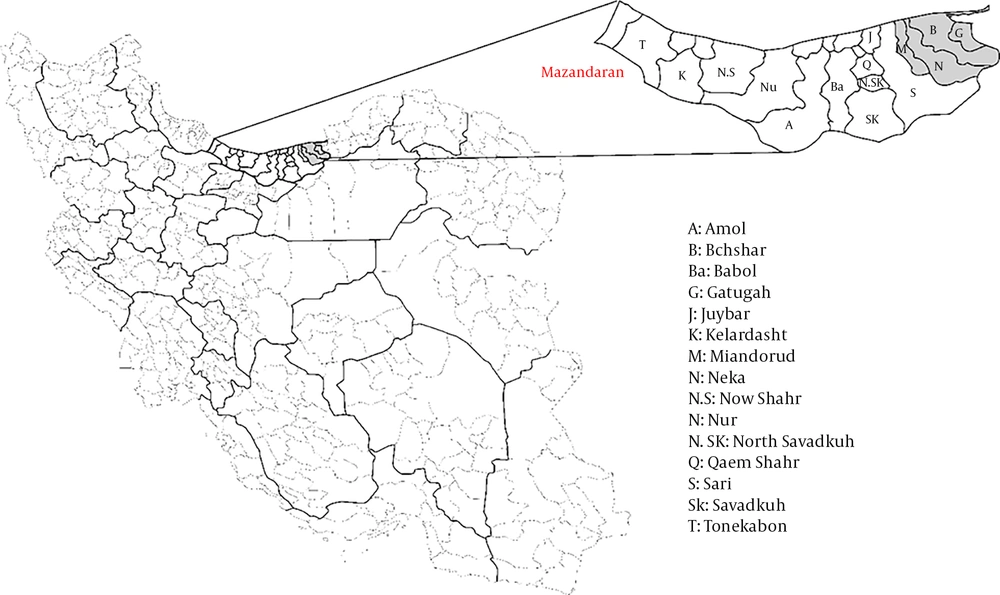
Study Location: This cross-sectional seroepidemiologic study was carried out in rural areas of four cities in the Mazandaran Province, namely Behshahr, Galugah, Miandorud, and Neka (Figure 1). Mazandaran is one of the coastal provinces of Caspian Sea with an area of about 23766.4 square kilometers. It is one of the most densely populated provinces of Iran, with a population of 3,283,582 according to a general census in 2016. This province has a moderate, semi-tropical climate. According to the general census, the maximum and minimum temperatures are 40.2°C and -19°C in this province, receptively.
Sample Size: The proportion of serum-positive vs. serum-negative individuals within the categories of each potential risk factor was considered to be 0.36 and 0.25, respectively (18). Given type I error of 0.05 and expected power of 0.8, the estimated accrual number was 448 individuals.
Sample Collection: From September 2018 until November 2018, a total of 448 participants were chosen by random sampling from the general population of rural areas of Behshahr, Galugah, Miandorud, and Neka. The inclusion criteria were as follows: 1) being Iranian; and 2) being a native and long-time permanent resident of study area. After receiving written informed consent from each participant, 10-mL blood samples were collected. Then, the sera were isolated and stored at -20°C before being sent to the Department of Arboviruses and viral hemorrhagic fevers (National Reference Laboratory) for serological testing. The demographic, epidemiological, and clinical data of the participants were also documented in a data gathering forms. The sampling was done in healthcare facilities and health centers in rural areas. The proportional size of sampling and number of samples was considered in all centers. The expected power of this study was considered as 80%.
The present study was carried out according to the Declaration of Helsinki and relevant national health regulations; all protocols of this study were approved by the Research Committee of Pasteur Institute of Iran (code: 565).
ELISA Assay: The serum samples were analyzed for the presence of anti-TBEV IgG antibodies, using the commercial Anti-TBEV ELISA (IgG) Kit (EUROIMMUN, Germany), based on the manufacturer’s instructions. According to the kit manual, the test showed 100% specificity and sensitivity. To interpret the results, the ratio of extinction coefficient of each serum sample to the extinction coefficient of calibrator 2 was determined. A sample with a ratio of ≥ 1.1 was regarded as positive for TBEV IgG. On the other hand, a sample with a ratio of ≥ 0.8 to < 1.1 was considered as borderline, while a sample with a ratio of < 0.8 was considered negative. Samples with borderline or positive results were considered as reactive.
Statistical Analysis: All data were analyzed in SPSS version 16 (SPSS Inc., Chicago, IL, USA). To check for the mechanism of missing in our dataset, we created dummy variables (1 = missing, 0 = observed). We then run t-tests and chi-square tests between the dummy variable and other variables in the dataset to see if the missingness is related to the values of other variables. We diagnosed missingness to occur completely at random (MCAR). Given the rate of missingness in our dataset was below 1%, we simply omitted missing values in our analyses. Chi-square test was used to analyze the correlation between categorical variables and infection. Independent sample t-test was also applied to examine the association between age and infection. P values ≤ 0.05 were considered statistically significant.
4. Results
A total of 448 healthy participants (62.8% female), with the mean age of 45.4 years (SD ± 16.9,), were investigated for the presence of anti-TBEV IgG antibodies using the ELISA assay and 16 (3.6%) sera samples were found to be reactive. As can be seen in Figure 1, among reactive cases, six (37.5%) belonged to Miandorud, five (31.2%) to Neka, three (18.8%) to Behshahr, and the remaining two (12.5%) to Galugah. No significant association was observed between study area and reactive cases (P = 0.95).
Table 1 demonstrates the correlation of demographic, epidemiological, and clinical features with seropositivity. Although there was no significant association between the reactive results and occupation, the majority of reactive cases were collected from individuals, who were involved in farming/animal industry-related jobs (33.3%) or were housewives (33.3%). The findings showed that keeping the livestock in close proximity to the household had a significant correlation with seropositivity, as reported in 75% of reactive cases. Similarly, a statistically significant association was observed between seropositivity and having livestock in house (P = 0.040).
| Characteristics | TBEV Seronegative (N = 432) | TBEV Seropositive (N = 16) | OR | 95% CI | P Value |
|---|---|---|---|---|---|
| Mean age | 45.4 ± 16.9 | 49.0 ± 14.9 | 1.0 | 0.9, 1.1 | 0.396 |
| Gender (female) | 265 (62.8) | 8 (50) | 1.7 | 0.6, 4.6 | 0.305 |
| County of residency | |||||
| Galugah | 101 (23.4) | 2 (12.5) | 1 | - | - |
| Behshar | 143 (33.1) | 3 (18.8) | 1.1 | 0.2, 6.5 | 0.950 |
| Neka | 95 (22.0) | 5 (31.2) | 2.7 | 0.249 | 0.5, 14.0 |
| Miandorud | 93 (21.5) | 6 (37.5) | 3.3 | 0.154 | 0.6, 16.6 |
| Occupation | |||||
| Retired | 15 (3.6) | 0 (0) | 1 | - | - |
| Housewife | 201 (48.4) | 5 (33.3) | 0.3 | 0.03, 3.2 | 0.350 |
| Farming/animal industry related jobs | 108 (26.0) | 5 (33.3) | 0.6 | 0.1, 6.0 | 0.702 |
| Employee/indoor related jobs | 52 (12.5) | 3 (20.0) | 0.8 | 0.1, 8.4 | 0.858 |
| Free lancer | 29 (7.0) | 1 (6.7) | 1.0 | 0.1, 12.0 | 1.000 |
| Worker | 10 (2.4) | 1 (6.7) | 1.4 | 0.1, 25.1 | 0.819 |
| Keeping livestock in house | 202 (46.8) | 12 (75) | 3.3 | 1.1, 10.5 | 0.040 |
| Consumption of local unpasteurized dairy | 387 (90.4) | 16 (100) | 1.6 | 0.2, 12.3 | 0.194 |
| History of tick bite/ tick squish | 62 (14.6) | 7 (43.8) | 4.5 | 1.6, 12.6 | 0.002 |
| History of traveling abroad | 148 (34.5) | 6 (37.5) | 1.1 | 0.4, 3.2 | 0.804 |
aValues are expressed as mean ± SD or No. (%).
A history of tick bite or tick squish on bare hands was recorded in 14.6% of negative cases, while this rate was significantly higher among reactive cases (43.8%) (P = 0.002).
All reactive cases reported a history of consuming local unpasteurized dairy, although the difference was not significant (P = 0.194). None of the cases reported a history of vaccination against TBEV, Japanese encephalitis virus, or yellow fever virus. Six (37.5%) out of 16 reactive cases had a history of travel to Iraq or Saudi Arabia for pilgrimage purposes.
5. Discussion
This is the first study providing evidence of TBEV seropositivity in Iran. Anti-TBEV IgG antibodies were detected in the rural population of Mazandaran Province in Northern Iran. In line with previous studies, we did not find any correlation between the seropositivity of TBEV and age or gender (19, 20). Despite the absence of a significant difference, farmers and or workers in animal industries (33.3%), and housewives (33.3%) displayed the highest seropositivity rates. It is well established that certain occupations, such as farming and livestock industry-related jobs, are accompanied by the increased risk of exposure to zoonotic vector-borne infections, like TBE (21, 22). In addition, in rural areas, housewives are at an increased risk of zoonotic diseases due to their involvement in various high-risk activities, such as working in farms, animal breeding, milk collection and processing, and meat handling of slaughtered livestock (23, 24). In one study on residents of rural areas in Sinop (Turkey) near Iran, the IgG positivity was detected in 2.9%, which were not found statistically significant with gender, age, occupation, and history of tick bite (25). In another study in Italy, the seropositivity against TBE virus was reported 0.6% (26). In a seroepidemiologic study in Poland, the mean percentages of seropositive samples in forestry workers and in farmers were reported 19.8% and 32.0%, respectably (27).
Therefore, like other well-known high-risk groups, housewives should also be warned about the risk of occupational exposure to zoonotic pathogens. In our study, all positive cases had consumed local unpasteurized dairies, and 75% had kept livestock at home. TBEV infection following the consumption of unpasteurized dairy products has been reported in the literature. According to previous reports, alimentary transmission of virus via infected dairy products accounts for 1% of TBEV infections. In addition, outbreaks of TBE after drinking raw milk have been reported in some endemic countries (13, 28-31).
TBEV infection in livestock is subclinical, and viral shedding from the livestock milk may continue for up to 19 days post-infection (32). Generally, alimentary transmission of TBEV is uncommon. However, consumption of raw milk and unpasteurized dairy products, as a daily routine, is becoming more popular (29). Therefore, training and informing people about the health-threatening consequences of consuming non-pasteurized dairy products can be an efficient and cost-effective strategy for reducing the transmission of TBEV and other zoonotic pathogens (33).
In the present study, we found a significant association between tick bite/tick squish and TBE seropositivity, as tick contact was almost three times more common in reactive cases, compared to negative cases. Despite the fact that the main vector for TBEV (i.e., I. ricinus) has been detected in the Mazandaran Province (16), to the best of our knowledge, there have not been any attempts to investigate TBEV infection in ticks.
There are several lines of evidence regarding the presence of I. ricinus in Northern Iran. In 2006, a high infestation rate of 86% with I. ricinus was reported in the cattle of Mazandaran Province (among 696 ticks) (34). Another study conducted in 2007 - 2008 showed that 2.3% of livestock in Mazandaran and Guilan provinces were infested with I. ricinus (among 1720 ticks) (35). Moreover, in 2008, Youssefi et al. (17), reported an infestation rate of 27% (among 798 ticks) with I. ricinus in the cattle and sheep of Mazandaran Province. In 2012, I. ricinus was found among questing ticks, as well as feeding ticks in Mazandaran Province (16). Also, in a recent report from Golestan, another Northern Province of Iran, I. ricinus was isolated from a Persian leopard (36). In comparison with other tick species, survival of I. ricinus is highly sensitive to humidity and temperature (37, 38). Close proximity to the Caspian Sea and presence of large and dense woods provide favorable conditions for the maintenance of I. ricinus populations.
It is well documented that there is uncertainty about the accuracy of positive results on serological tests, such as ELISA, owing to the antigenic cross-reactivity among flaviviruses (39, 40). Consequently, a supplementary virus neutralization assay is needed to confirm the ELISA results. Our findings, therefore, should be interpreted with caution, given the fact that we could not perform a virus neutralization assay. However, the used ELISA kit showed maximum specificity and sensitivity (100%) according to the manufacturer’s protocols. To provide definitive evidence for circulation of TBEV in Mazandaran, further research for detection of viral RNA in ticks (both feeding and questing) and milk of livestock seems necessary. Although no case of TBE has been reported in Iran, it is not clear whether this condition persists for a long time. However, a recent increase in both the incidence and distribution of TBE has been reported due to changes in climate, ecology, and social behaviors (41), which are factors that have influenced Iran.
This study has some limitations, including its limited study area and not including vectors and possible reservoirs.
In conclusion, this study provides evidence on the circulation of TBEV in Northern Iran, where climatic conditions, presence of Ixodes ticks, and variability of mammalian hosts might contribute to TBEV establishment. Integrated research on tick vectors, animal reservoirs and humans would be recommended to provide more information about epidemiology of TBEV in this area.