1. Background
Multidrug-resistant tuberculosis (MDR-TB) is a public health crisis. Indonesia is one of 30 countries with the highest MDR/RR-TB cases in the world, with the number of incidences of MDR/RR TB 23,000 and the percentage of MDR TB is 91% (1). Drug-resistant tuberculosis (DR-TB) cases in Dr. Soetomo Hospital were 90 cases in 2012, 143 cases in 2013, 142 cases in 2014, and 140 cases in 2015 (2). The treatment of MDR-TB is challenging, especially for patients with comorbidities. The incidence of diabetes mellitus (DM) is increasing globally (3).
People with diabetes mellitus (DM) have a higher risk for DR-TB infection. Among patients with MDR-TB, DM is a common comorbidity, which was reported to be correlated with an increased risk of treatment failure and death in MDR-TB-treated patients and followed for 8–11 years (4). Comorbidity of DM in MDR-TB treatment causes the worse adverse effect and treatment outcomes, increases the cost of treatment, promotes the MDR-TB transmission, and can also generate extensively drug-resistant TB (XDR-TB) (5). Diabetes mellitus may affect TB response treatment and cause more adverse effects. In some cases, adverse effects cause a changed DR-TB regimen and can also cause permanent discontinuation of the drug used due to adverse effects (6).
A greater bacillary load at the baseline was found in patients with DR-TB having comorbidity of DM, which caused a longer time to culture conversion. Diabetes mellitus caused a change in drug absorption and impaired renal or liver function in drug clearance (7). It may be hypothesized that DR-TB with DM increased the risk of an unfavorable outcome. Many studies from different countries suggested that there is a significantly higher risk of unfavorable outcomes in patients with DR-TB and DM (3, 4, 8, 9), while other studies reported different results that there was no correlation (10-13). Patients with DM have a higher risk of developing serious adverse effects of DR-TB treatment, such as nephrotoxicity and hypothyroidism (12).
2. Objectives
This study was conducted on DR Pulmonary TB (DR-PTB) patients to evaluate the effect of DM on specific adverse effects such as impaired renal function and audiology impairment, as well as to evaluate treatment outcome due to DR-TB regimens treatment to improve the treatment strategy of DR-TB with DM.
3. Methods
3.1. Study Design
This was a retrospective study conducted from 2016 to 2017. Study subjects were DR pulmonary TB patients in Dr. Soetomo Hospital, as a referral hospital for TB and DR-TB in east Indonesia. Patients with DR-TB receive DR-TB regimens containing kanamycin with normal renal and audiology functions at baseline tests were included in this study. Patients with DR-TB received nephrotoxic and ototoxic drugs, and patients with HIV comorbidity were excluded from this study.
3.2. Operational Definition
HbA1c >7 was used to define DM. The adverse effects in this study were impaired renal function (increased serum creatinine) and audiology impairment. Treatment outcomes were divided into favorable outcomes and unfavorable outcomes. Favorable outcomes were cure and treatment completion, while unfavorable outcomes were treatment failure, loss to follow-up, and death.
3.3. Audiometry and Renal Function Test
Serum creatinine levels were examined monthly as laboratory monitoring. Audiology impairment was based on an audiometry test using an automatic machine. Increased serum creatinine was above the normal range (0.6 – 1.3 mg/dL) according to the standard in Dr. Soetomo Hospital. Ototoxicity is diagnosed by comparing an initial audiogram -ideally obtained before initiation of ototoxic drugs- with hearing thresholds using serial audiograms (14). Audiology impairment was defined based on hearing-value according to American Speech-Language-Hearing Association (ASHA), audiometry test above 25 dB.
3.4. Data Analysis
Adverse effects and treatment outcomes were compared between DR-TB patients with DM and without DM. Adverse effects and treatment outcomes were analyzed using unadjusted relative risk (RR) and 95% confidence interval (95% CI). SPSS 21.0 was used for all statistical analyses.
4. Results
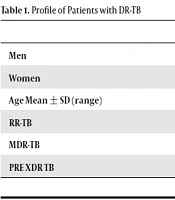
There were 202 patients with DR-TB in this study, consisted of 82/202 (40%) DR-TB patients with DM, and 120/202 (60%) of DR-TB patients without DM. Mean age of DR-TB patients with DM was 47.26 years old, and DR-TB patients without DM was 38.31 years old (Table 1).
| With DM, No. (%) | Without DM, No. (%) | Total | |
|---|---|---|---|
| Men | 40 (36.4) | 70 (63.6) | 110 |
| Women | 42 (45.7) | 50 (54.3) | 92 |
| Age, mean ± SD (range) | 47.26 ± 8.818 (26-68) | 38.31 ± 11.061 (16-73) | 41.94 ± 11.098 (16-73) |
| RR-TB | 5 (41.7) | 7 (58.3) | 12 |
| MDR-TB | 66 (39.1) | 103 (60.9) | 169 |
| PRE XDR TB | 11 (52.4) | 10 (47.6) | 21 |
There were 28/82 (34.1%) DR-TB patients with DM and 20/120 (16.7%) DR-TB patients without DM who experienced increased serum creatinine. Patients with DM have a risk ratio (RR) for increased serum creatinine with RR 2.049 (95% CI: 1.242 - 3.379), as presented in Table 2.
| Increased Serum Creatinine | Normal Serum Creatinine | Total | RR (95% CI) | |
|---|---|---|---|---|
| With DM, No. (%) | 28 (34.1) | 54 (65.9) | 82 | 2.049 (1.242 – 3.379 |
| Without DM, No. (%) | 20 (16.7) | 100 (83.3) | 120 |
There were 22/82 (26.8%) DR-TB patients with DM and 19/120 (15.8%) DR-TB without DM experienced audiology impairment. Patients with DM have a risk ratio (RR) for increased serum creatinine, audiology impairment with RR 1.694 (95% CI: 0.982 - 2.925) as presented in Table 3.
Patients with unfavorable outcomes (treatment failure, loss to follow-up, and death) were 37/82 (45%) patients with DM and 46/120 (38%) patients without DM. Our study revealed that patients with DM have a risk ratio for unfavorable outcomes with RR 1.177 (95% CI: 0.847 - 1.636) as presented in Table 4.
| Audiology Impairment | Normal Audiology | Total | RR (95% CI) | |
|---|---|---|---|---|
| With DM, No. (%) | 22 (26.8) | 60 (73.2) | 82 | 1.694 (0.982 – 2.925) |
| Without DM, No. (%) | 19 (15.8) | 101 (84.2) | 120 |
| Unfavorable Outcome | Favorable Outcome | Total | RR (95% CI) | |
|---|---|---|---|---|
| With DM, No. (%) | 37 (45) | 45 (55) | 82 | 1.177 (0.847- 1.636) |
| Without DM, No. (%) | 46 (38) | 74 (62) | 120 |
5. Discussion
Of the 202 patients, there were 110 (54%) men and 92 (46%) women in this study. According to the WHO global TB report, there were 5.8 million men, 3.2 million women, and 1.0 million children who developed TB disease in 2017 (1). In Indonesia, TB is significantly more common among men than among women (15).
Aminoglycosides are often toxic to both the kidney (nephrotoxicity) and the inner ear (ototoxicity). Nephrotoxicity, however, is often reversible, while ototoxicity is generally permanent (16). Our study found that DM increases the risk for adverse effects and serum creatinine with RR 2.049 (95% CI: 1.242 - 3.379). Unregulated DM correlates with acute ketoacidosis and chronic complications, such as diabetic nephropathy, neuropathy, retinopathy, diabetic foot, and cardiovascular problems (17). Diabetes mellitus alters drug absorption and impairs renal or liver function in drug clearance (7). Amikacin, kanamycin, and other aminoglycosides are practically not metabolized by the human body and are excreted unchanged almost exclusively by glomerular filtration. Renal clearance may strongly affect the toxicity of aminoglycosides (18). Diabetes causes glomerular hyperfiltration, classically has been hypothesized to predispose to irreversible nephron damage, thereby contributing to initiation and progression of kidney disease in diabetes (19). A study in Mexico reported that the presence of DM was associated with an increased risk of serious adverse effects such as nephrotoxicity (OR = 6.5; 95% CI: 1.9 - 21.8) (12). The mechanisms by which aminoglycosides may alter the renal tubular function remain speculative. Available data suggest that the proximal tubular diseases (and acute kidney injury) might result from mitochondrial dysfunction. On the other hand, the loop of Henle/distal tubular injury may result from activation of the calcium-sensing receptor (20).
Our study demonstrated that audiology impairment in patients with DM was higher than patients without DM (26.8% Vs 15.8%). DM increased risk for audiology impairment. Torrico also reported that ototoxicity was higher in patients with DM compared to patients without DM (56% Vs 32%), with OR 2.8; 95% CI: 0.8 - 10.6 (12). The use of kanamycin in MDR-TB treatment causes the adverse effect of hearing loss in more than 25% of all patients in a prospective cohort (21). There are various theories of aminoglycoside ototoxicity, including oxidative stress and free radical formation (22), uptake and penetration of the aminoglycosides within the cochlear cells (23), and well as drug concentrations (24). The aminoglycosides used to treat DR-TB can cause irreversible hearing loss, as they destroy the outer hair cells in the cochlea. The exact pathophysiological mechanism is not entirely understood (25). Nevertheless, once the aminoglycosides are inside these cochlea cells, they start to generate reactive oxygen species, which is central to the destruction of these hair cells (22). Diabetes mellitus was significantly associated with ototoxicity in patients with DR-TB (26). A previous study reported that ototoxicity was found in 18/100 (18%) of patients with MDR-TB who received kanamycin. Ototoxicity was associated with comorbid conditions like DM and hypertension (27). Diabetes mellitus is closely linked to hearing damage. Both large and microscopic size blood vessels are affected in DM. Metabolic disorders, atherosclerotic changes, and microvessel diseases result in ischemia and hypoxia in neural tissues, leading to nerve damage. When such pathological changes involve the cochlea, and auditory nerve, cochlear and/or neural hearing loss follows (28).
Our study established that DM increased the risk for unfavorable outcomes with RR 1.177 (95% CI: 0.847 - 1.636). The unfavorable outcomes were 37/82 (45%) with DM and 46/120 (38%) without DM. Another study also reported that DM has a significant association (OR 3.578; 95% CI: 1.114 - 11.494) with the development of adverse effects. Diabetes mellitus is associated with treatment outcomes in pulmonary TB patients and adverse effects (29). Management of DR-TB is complicated. Second-line drugs (SLDs) used to treat DR-TB are less potent and costlier than first-line drugs (FLDs). Second-line drugs are also correlated with more adverse events and are less tolerated (30). Another study reported different results that DM was not associated with unfavorable outcomes in patients with DR-TB, while DR-TB and HIV co-infection, second-line drug resistance, and history of treatment in the private sector were more frequently associated with adverse outcomes (31). Diabetes mellitus did not correlate with DR-TB treatment outcomes, but DM in patients with DR-TB increased serious adverse effects to DR TB treatment, such as nephrotoxicity and hypothyroidism (12). Another study reported that DM correlated with the treatment outcome as well as adverse drug reaction incidence (29).
The limitations of the study are as follows: comorbidity of DM was defined based on baseline examination, and there were no data of regulated and non-regulated DM. Medical records also did not mention whether patients with DM were insulin-dependent. We also did not include variables which might also have an association with adverse effect and treatment outcomes in patients with DR-TB.
5.1. Conclusions
Diabetes mellitus increases the risk of adverse effects, serum creatinine, and audiology impairment. It also increases the risk of unfavorable treatment outcomes in patients with DR pulmonary TB who receive DR-TB regimens containing kanamycin.