1. Background
Toxoplasma gondii, an apicomplexan protozoan parasite, infects approximately one-third of the world population. The obligate intracellular parasites can infect a wide variety of warm-blooded animals, including humans. Direct contact with wandering cats, due to transmission of oocytes, and eating raw or semi-cooked meat because of transmission of tissue cysts have always been among the risk factors of developing toxoplasmosis. It is commonly believed that toxoplasmosis does not manifest serious illness, especially in immunocompetent patients (1). Immunocompromised individuals and pregnant women with no history of toxoplasmosis are at a higher risk of serious complications of this infection, including encephalitis and dissemination of replicating tachyzoites (2).
According to the literature, 15% of infertility cases in males are due to infections of the reproductive system (3). Many bacteria, viruses, parasites, and yeasts lead to failure in fertility in different ways (4). T. gondii is one of the microorganisms which can be involved in the infection of the male reproductive system. Studies have indicated that chronic toxoplasmosis in female rats resulted in endometriosis, impaired function of ovaries, impaired folliculogenesis, uterine atrophy, and even diminished weight of gonads (5, 6). Studies on protozoan infections in the male reproductive system are rare, and they are generally presented in case reports (7). Defects in the male reproductive system by T. gondii have been claimed in various studies; for example, a systematic review suggested the possibility of T. gondii damage in the testis through secondary hypogonadism by changes in the hypothalamic-pituitary axis (7). A number of studies have indicated that T. gondii exists in the seminal fluid of humans, goats, and pigs (8-10). Recent records through an experimental study showed that this intracellular parasite affected all sperm parameters, including motility, concentration, and morphology (11). In another study, although there was a significant reduction of spermatozoa in male mice, no sterility was reported one month after T. gondii infection (12). Transmission of the parasite from infected male animals to female ones has occurred through sexual activity, too (13-16). Based on the above explanation and due to the high prevalence of toxoplasmosis in human populations, it is necessary to carry out studies on the involvement of this protozoan in the male genital tract.
2. Objectives
As T. gondii is common in humans and it is necessary to evaluate the effects of this parasite on the male genital system, this study aimed to investigate the effect of T. gondii on sperm parameters and hormone levels in the experimental model of toxoplasmosis in male rats.
3. Methods
3.1. Animals and Infection
A total of 80 male Wistar rats within the weight range of 206 - 275 g were obtained from the animal house of Hamadan University of Medical Sciences, who were randomly divided to control and infected groups. A total of 107T. gondii tachyzoites RH strain achieved by intraperitoneal serial passage previously infected mice every 3 - 5 days (17) were injected into peritoneum of 40 rats of the infected group simultaneously, and the rats of the control group were inoculated with normal saline at the same volume (18). The rats were kept in 12:12 dark/light cycles at 23 ± 1°C and 55 - 60% humidity. The research was performed according to the principles and ethical considerations of working with laboratory animals as confirmed by the ethics committee of Hamadan University of Medical Sciences (code: REC.1396.125.IR.UMSHA). Following inoculation on days 10 - 80 within 10-day intervals, five rats from the infected group and five rats from the control group were first weighed and then anesthetized and sacrificed according to ethical considerations. Blood samples were taken from the inferior vena cava. Also, the testis and epididymis were removed instantly (19).
3.2. Toxo-IgG ELISA Test
To confirm toxoplasmosis contamination, anti-toxoplasmosis IgG level was tracked in the serum of rats according to manufacturer’s instructions (ZellBio, ZB-Q16312S-R9648). Eventually, the optical densities were measured at 450 nm with an ELISA reader (1).
3.3. Sperm Parameters Assessment
Sperm parameters (number, motility, morphology, and viability) were indicated according to World Health Organization (WHO) protocol (20). For this purpose, after sacrificing the animal, the left cauda epididymis was removed and cut into different pieces. It was placed in a Petri dish containing Ham’s F10 medium (Sigma-Aldrich, St. Louis, USA) for 15 min at 37°C. Approximately 10 μL of diluted sperm was transferred to the counting hemocytometer slide and allowed to stand for 5 min. The cells which settled during this time were counted by a light microscope (Zeiss, Munich, Germany) at ×200 magnification (21, 22).
Motility of sperms was evaluated by counting the percentage of motile sperms with magnification of 400X. The percentage of viable sperms was determined by counting the spermatozoa excluding vital stain (Eosin 0.5%) (23). The abnormal sperms such as headless, double head, pinhead, coiled tail, bent neck sperms, and multiple abnormalities were also counted per 100 (24).
3.4. Histological Evaluation
Each testis was weighed and fixed in formalin 10%, then embedded in paraffin. Five micron slices were prepared from the testis paraffin blocks and stained by H&E staining. Next, spermatogonia, primary spermatocytes, spermatids, Leydig cells, and Sertoli cells were counted in 25 seminiferous tubules. In the same section, area, circumference, and diameter of seminiferous were measured under the light microscope equipped with Motic Moticam 2000 2.0 Pixel camera (Motic Kowloon, Hong Kong) (25).
3.5. Serum Testosterone, FSH, and LDH
Serum concentration of testosterone, LH, and FSH was performed using ELISA test according to the kit instructions produced by Pishgaman Sanjesh Company.
3.6. Data Analysis
All statistical analysis was performed using SPSS software V.16. (IBM Corp., Armonk, NY.USA). The variables were analyzed by Mann-Whitney and Kruskal-Wallis tests. All data were expressed as mean ± standard deviation (SD). The statistical level of significance was set at P < 0.05.
4. Results
4.1. ELISA IgG Test
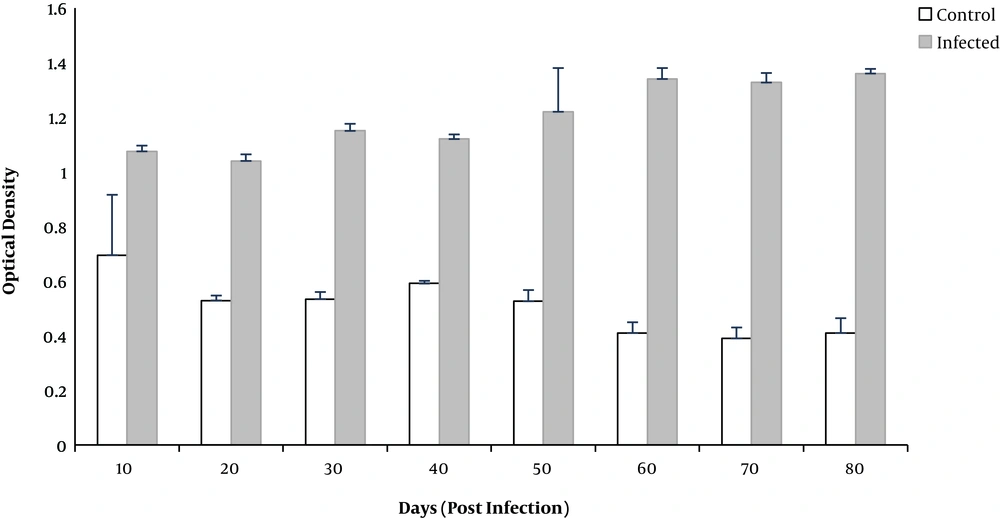
Investigation of the mean serum IgG indicated that this level after inoculation in the infected group was positive. Sera of control rats were negative for T. gondii IgG (Figure 1).
4.2. Weight Parameters
Bodyweight increased during the study days. However, the mean body weight on days 20 and 60 in the infected group showed a significant reduction in comparison to the control group (P = 0.02)
Increased testis weight was observed in the infected group in comparison to the control group across all days of the study. Differences were significant on days 40 and 70 (P = 0.01 and P = 0.04, respectively).
4.3. Sperm Parameters
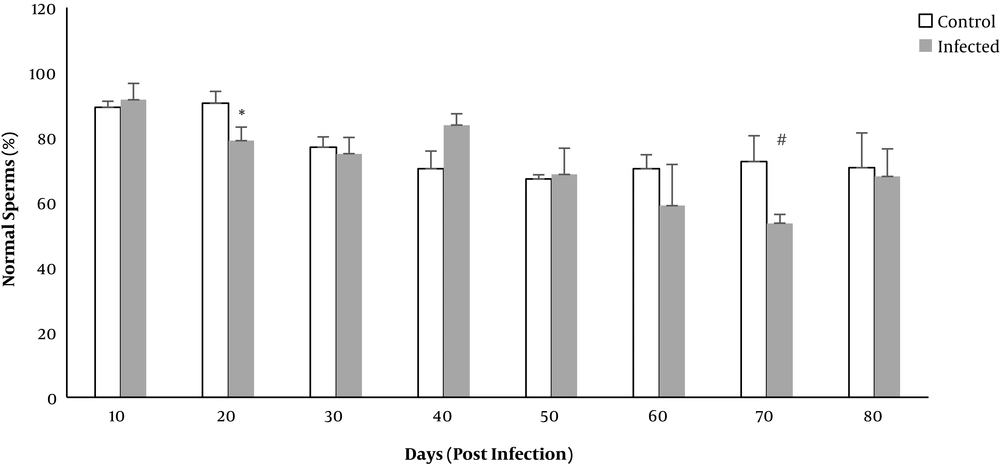
The percentage of normal sperms decreased in both control and infected groups. The mean percentage of normal sperms in terms of morphology decreased significantly on days 20 and 70 in the infected rats (P = 0.008 and P = 0.009, respectively) (Figure 2).
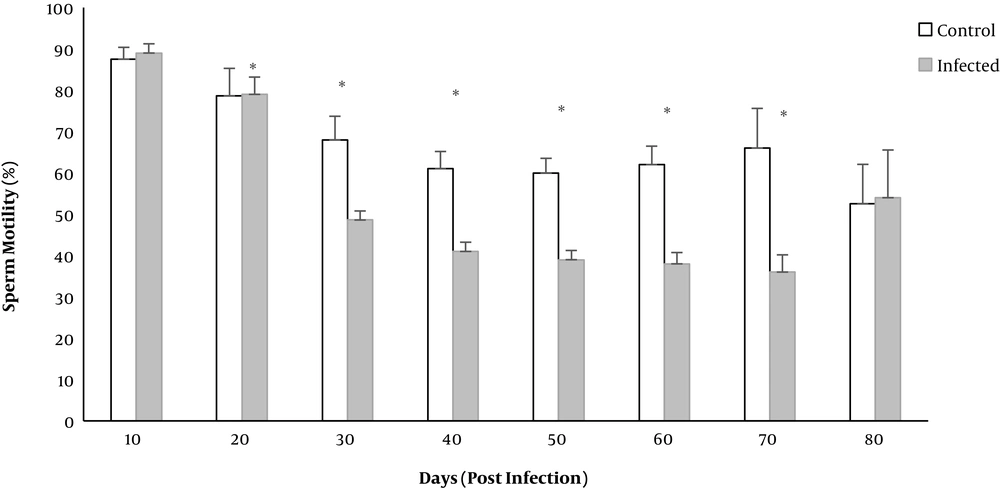
The mean percentage of motile sperms significantly decreased in the infected group on days 20 to 70 (P < 0.01) (Figure 3).
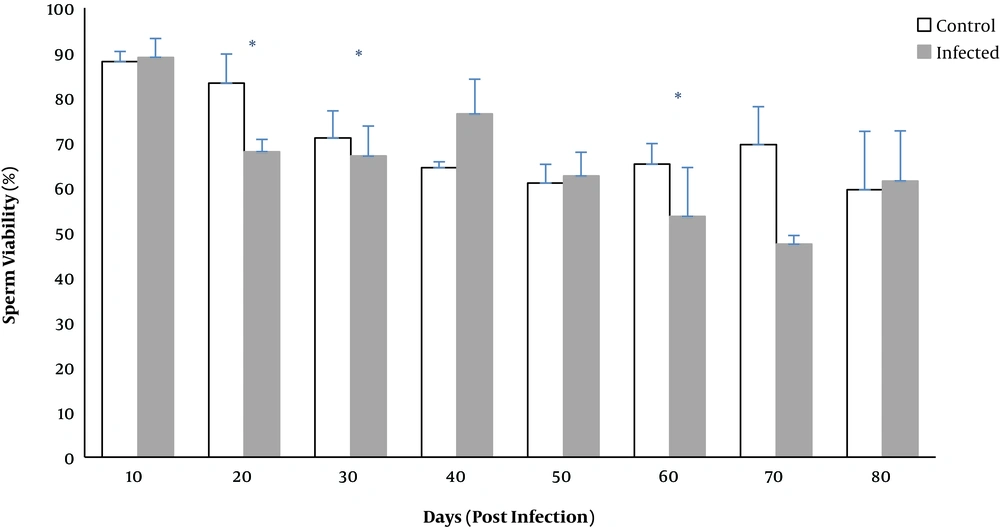
The percentage of viable sperms significantly decreased on days 20, 30, and 60 (P < 0.02) (Figure 4).
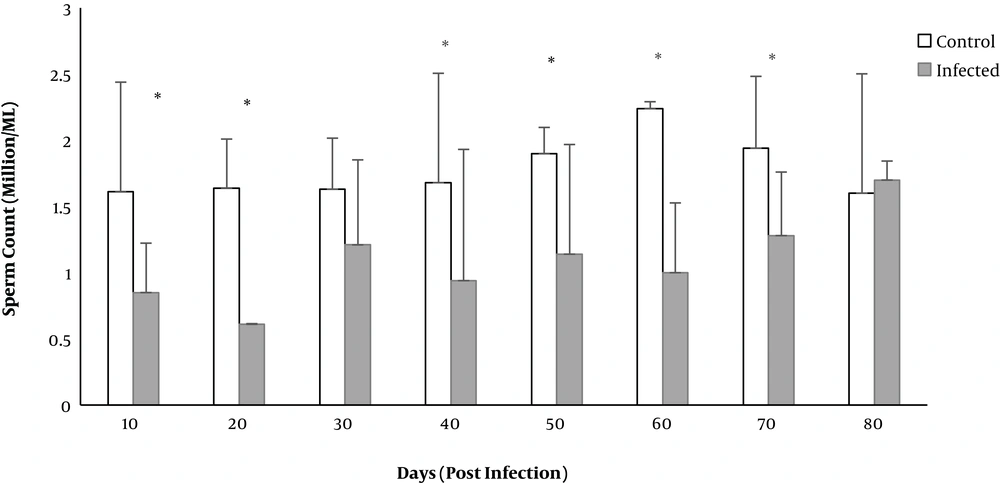
The total sperm count in the infected group significantly decreased on days 10 to 70 except for day 30 (P < 0.05) (Figure 5).
4.4. LH, FSH, and Testosterone Hormones
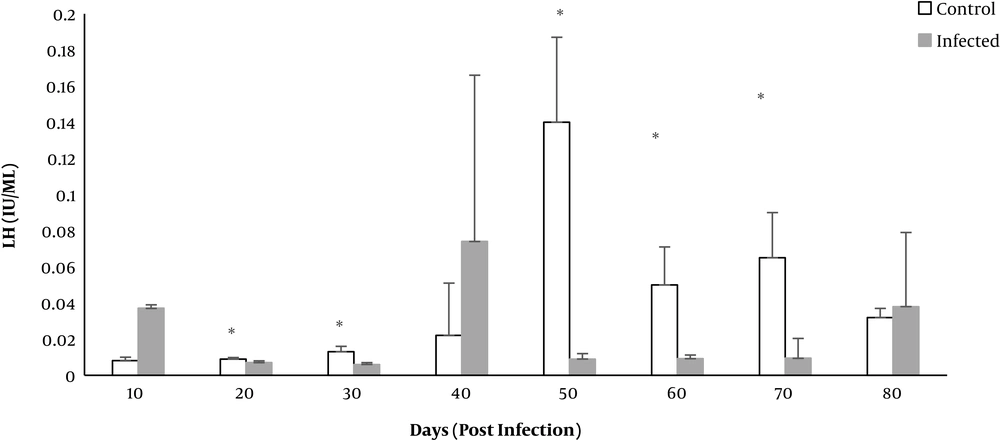
The level of LH hormone on days 20 - 70 (except for day 40) was significantly lower in the infected group compared to the control group (P < 0.01) (Figure 6).
The level of FSH and testosterone hormones was higher in the infected group compared to the control group across all days of the study. However, no significant difference was found.
4.5. Histological Evaluation
Reduction in the mean number of Leydig cells was observed in Toxoplasma infected rats. However, a significant difference was reported on day 20 (P = 0.026). The number of Sertoli cells exhibited a significant decline on days 50 and 60 (P = 0.028).
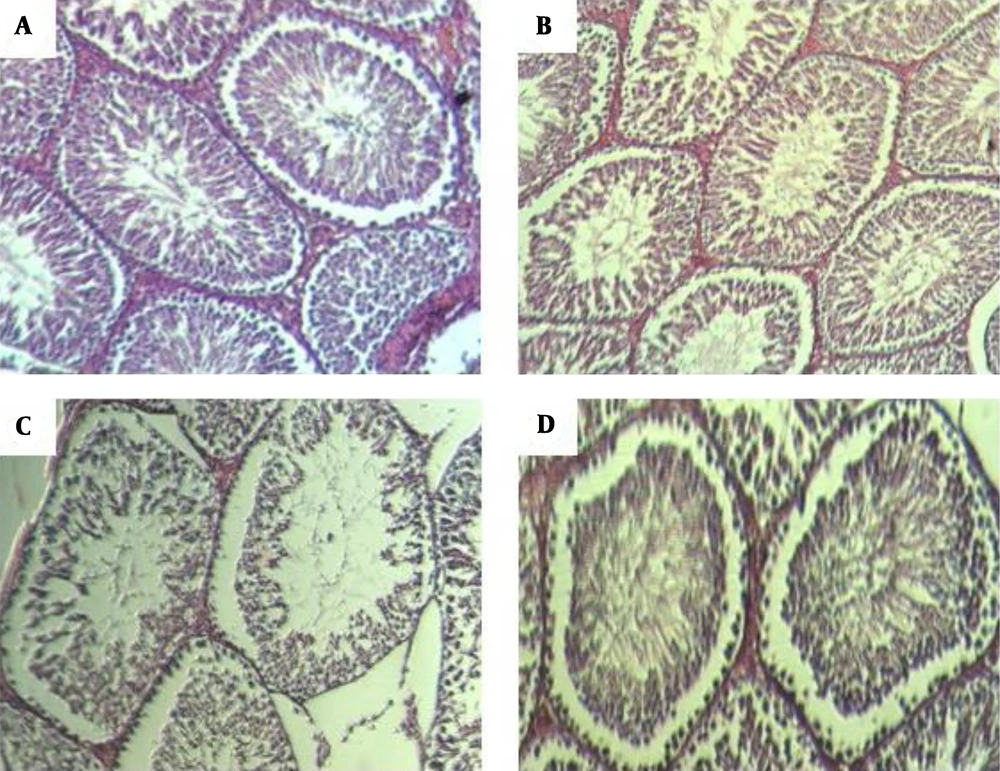
The number of seminiferous layers significantly decreased on day 20 in the infected group compared to the control group (P = 0.034). Spermatids were significantly lower in the infected group on day 40 (P = 0.015). There was no significant difference in the number of spermatocytes. Seminiferous thickness increased across the study in the infected group. The difference was statistically significant on days 40 and 60 (P = 0.014 and P = 0.009) (Figure 7).
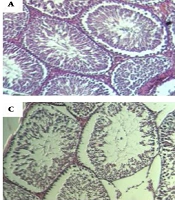
Histological structure of rat testis as assessed in paraffin sections. Hematoxylin and eosin staining (A) control group with normal arrangement of spermatozoa. Irregular arrangement of seminiferous tubules on days 40 band 60 (B) post infection. Germ cells were available but fewer in number. Vacuolization was apparent in the germ and sertoli cells on day 60 (C). Regeneration of seminiferous was almost normal on day 70 (D).
5. Discussion
The prevalence of infertility in the world is around 13%. Lack of a significant improvement in seminal parameters is the most common cause of infertility in males (19). In our study, sperm parameters along with LH hormone reduction were observed in Toxoplasma infected rats. As a model of human toxoplasmosis, rats are the most similar example. Furthermore, they are much more resistant to T. gondii infection compared to mice (26). In the present study, the mean body weight of rats increased, but a significant decrease on days 20 and 60 was detected. The mean weight loss on day 20 seems to be due to the severity of the infection; however, Tripsidis et al. reported no significant difference in body weight (11). In addition, the mean weight of testis increased in the infected group in comparison to the control. The increase remained significant on days 40 and 70. In the study by Terpsidis et al., no significant difference was observed among groups regarding the weight of testis (11). In the study by Abdoli on experimental toxoplasmosis, no significant difference was observed in the ratio of testis weight to the body weight (19). The possible reason for the elevation of testis weight may be due to inflammation, edema, or cell infiltration developed in response to parasites.
Based on investigations, sperms count, motility, viability, and normal morphology are the best factors for determining infertility (27). The current study revealed that toxoplasmosis could reduce sperm parameters, such as normal sperm morphology, sperm viability, and sperm count. The percentage of abnormal sperms, headless, tailless, or double head sperms increased significantly on days 20 and 70. Significant reduction in motile sperms was observed on days 20 to 70, and the number of sperms decreased on days 10 to 70 except for day 30. Consistent with the present study, Abdoli found that toxoplasmosis could disrupt the function of the reproductive system of male rats, including enhancement of abnormal or dead sperm and diminution of sperm count or motile sperms (19). Similar results were reported by Trepsidis et al., where sperm parameters decreased in an experimental study of rat toxoplasmosis (11). Accordingly, it can be concluded that T. gondii affected the reproduction system of male rats. Components similar to LPS in bacteria such as glycosyl phosphatidylinositol (GPI) have been demonstrated in T. gondii, which activate macrophages and natural killer cells to release ROS and nitric oxide; excessive amount of inflammatory cytokines may contribute to oxidative damage to lipids, protein, and nucleic acids (28). Elevated ROS production can lead to mitochondrial DNA damage, progression of the systemic inflammatory response, and cell death as well as sperms death (29). Destructed sperm membrane, lack of motility, and sperm abnormalities due to excessive load of ROS were described earlier (30).
Since T. gondii caused behavioral and neurological changes in human and animal hosts, the development of hormonal changes is also possible (31). A significant decline in LH level on days 20 - 70 of the study (except for day 40) was detected in the Toxoplasma infected rats. LH level in urine of mice infected by T. gondii significantly declined in the study by Dvorakova (12). In spite of the numerical reduction in testosterone and FSH, no significant difference was observed. However, studies by Flegr and Hodkova indicated that toxoplasmosis in male and female rats resulted in diminished testosterone levels (32, 33). Since LH plays a significant role in the development and differentiation of germ cells and controls the extent of testosterone secretion, thus it can be concluded that reduction of LH level can cause impaired spermatogenesis (34). FSH is also one of the hormones which is responsible for regulating the function of the genital system. Gonadotropins (LH and FSH) regulate the entire process of spermatogenesis. Infective agents, by increasing the production of stress hormones and inhibiting the hypophysis-hypothalamus control axis, lead to diminished LH and FSH, and in turn, reduced spermatogenesis (35).
In the study by Dvorakova (2014), the mean number of spermatids and secondary spermatocytes layers decreased in the infected mice as compared to the healthy ones. However, the number of Sertoli cells and diameter of seminiferous tubules increased (12). Similar results were obtained in the present study; the number of Leydig cells, Sertoli cells, and spermatids, as well as the diameter of seminiferous tubules, decreased. The thickness of the seminiferous tubules was increased in the infected group, but this ascending trend was statistically significant on days 40 and 60. However, whether toxoplasmosis causes changes in the number of cells or reduction of diameter, specifically by affecting the hormonal system, immunological reactions, or oxidative processes, is still unclear. Previous studies suggested that most drugs or toxins cause changes in histomorphometric parameters of the testis through developing oxidative stress and inducing apoptosis (36, 37). For example, Fathi 2018 found that silver nanoparticles decrease the parameters of sperm and cause a significant reduction in the number of spermatogonia, Sertoli cells, and Leydig cells (21). Some researchers believe that because of the pathogenicity of toxins or microorganisms in testis, different changes developed in the hypothalamus-hypophysis-testicle axis leading to diminished activity of Sertoli cells, damage, and atrophy in Leydig cells, and inhibited the synthesis of testicular steroids, and in turn, diminished production of endogens; all these eventually lead to stimulation and developmental changes in the germinal epithelium of the seminiferous tubules (25).
5.1. Conclusion
Our results showed that T. gondii infection can lead to diminished fertility parameters but not sterility. Since, at the end of the study, most parameters approached the mean value of the control group, it can be concluded that the effect of the parasite on male fertility factors is temporary, and as the disease is controlled by the immune system, the infertility conditions may be reversible. On the other hand, day 20 could be considered as the acute day of infection because most parameters of sperm analysis in the infected group were affected. Investigations in this study showed that this parasite does not lead to pathologic damages in the testis.