1. Background
It is estimated that the burden of the human immunodeficiency virus (HIV)- hepatitis C virus (HCV) co-infection affects almost 5 - 7 million people worldwide (1, 2). HIV accelerates HCV-related fibrosis progression and is resulted in a higher rate of liver decompensation compared with HCV mono-infection (3). Severe liver disease caused by HCV has become a leading cause of morbidity and mortality in HIV-positive patients (4). HCV treatment with pegylated interferon (PEG‐IFN) plus ribavirin (RBV) represented sustained virological response (SVR) rates of more than 30% among HIV-HCV patients (5). Although the new generation of direct-acting antiviral agents (DAAs) has caused an improvement in the treatment of HCV in HIV-HCV co-infected patients, simultaneous treatment of HIV and HCV remains complicated with challenges, including drug-drug interactions between DAAs and antiretroviral therapy (ART) (6). Daclatasvir (DCV), a pan-genotypic non-structural protein 5A (NS5A) inhibitor, in combination with sofosbuvir (SOF), the NS5B polymerase inhibitor, have been shown effective in HIV-HCV co-infected patients with different genotypes (7). There are no absolute drug-drug interactions between SOF/DCV and ARTs. However, dose adjustment is needed for some cases (6). In the ALLY-2 phase III study, 97% of HIV-HCV co-infected patients receiving 12 weeks of SOF/DCV achieved SVR12 across a broad range of ARV regimens (8). Nevertheless, limited data exist on the efficacy and safety of DCV/SOF in the treatment of HCV/HIV co-infected patients in a real-world setting.
2. Objectives
In this study, we assessed the efficacy and tolerability of SOF/DCV ± RBV in patients with HIV-HCV co-infection with or without cirrhosis in the real-life setting in Iran.
3. Methods
3.1. Study Design and Participants
This study was an open-label trial of patients with HIV-HCV co-infection who visited the Labbafinezhad Hospital, Tehran, Iran, between June 2018 and July 2019. Patients were categorized according to ART. The first group receiving the lopinavir-containing regimen (a PI regimen) was treated with DCV 60 mg/SOF 400 mg (Datex) once daily for 12 weeks. The 60 mg dose of DCV was adjusted to 30 mg for patients receiving atazanavir-containing regimen (a PI regimen), and to 90 mg for those receiving efavirenz-containing regimens (an NNRTI regimen). Adding RBV or extending the treatment duration up to 24 weeks may occur at the physician’s discretion. Adding RBV to the patient’s treatment regimen might occur if the patient had cirrhosis, advanced fibrosis, or history of HCV treatment. Patient’s demographic data, risk factors, serum HCV and HIV RNA, CD4 cell counts, HCV genotype, cirrhosis status, HCV treatment experience, results of laboratory tests, and liver enzymes before treatment, at the end of treatment, and 12 weeks after treatment recorded in the patient’s clinical records were reviewed. Cirrhosis status was determined based on clinical findings, laboratory results, biopsy, or Fibroscan (cirrhosis was determined as a METAVIR score of F4 or a liver stiffness value ≥ 14.6 kPa, and the advanced fibrosis was defined as a METAVIR score of F3 or a liver stiffness value ≥ 9.6 kPa but < 14.6 kPa).
3.2. Assessment
The goal of treatment was sustained virologic response 12 weeks after treatment (SVR12). Safety, CD4 count, and abnormality in laboratory tests and liver enzymes were also assessed.
3.3. Statistical Analysis
Descriptive statistics for continuous and categorical variables were expressed as median (range) and number (percentage), respectively. Statistical analyses were performed using the chi-square test for categorical variables through SPSS version 24.0. Statistical significance was considered as a P value of ≤ 0.05.
3.4. Ethics
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences and the Infectious Diseases and Tropical Medicine Research Center (Approval No.: Ir.sbmu.msp.rec.1398.010) in accordance with the Helsinki Declaration.
4. Results
4.1. Baseline Characteristics
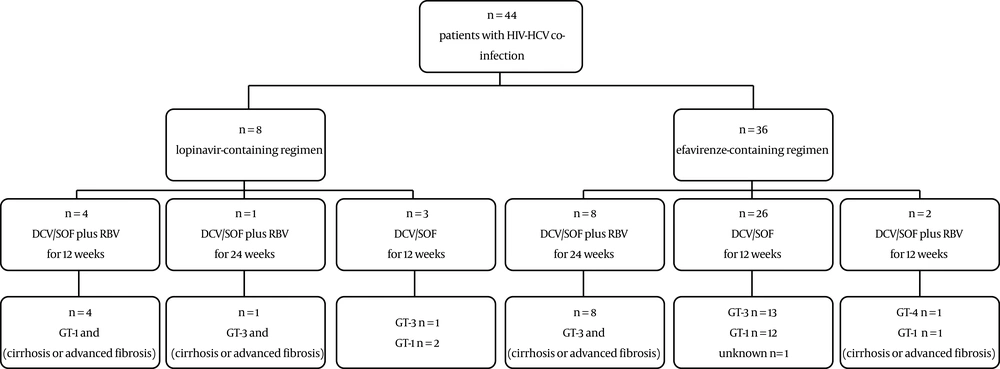
Of the 44 screened patients, 36 patients were on treatment with an NNRTI regimen (efavirenz) concomitant with either emtricitabine/tenofovir or zidovudine/lamivudine, and 8 patients were on a PI regimen (lopinavir/ritonavir) concomitant with the NRTI regimens, including zidovudine, lamivudine, tenofovir, and abacavir. The onset of HIV treatment varied from a few years prior to the HCV treatment to the onset of HCV treatment. Among patients on PI regimen, 4 cases with cirrhosis and GT-1 received DCV/SOF/RBV for 12 weeks, one case with cirrhosis, and GT-3a received DCV/SOF/RBV for 24 weeks, and three cases with either GT-1 or GT-3a received DCV/SOF for 12 weeks. Patients on the efavirenz-containing regimen were as follows: eight cases with cirrhosis and GT-3a received DCV/SOF/RBV for 24 weeks, two cases with cirrhosis and either GT-4 OR GT-1 received DCV/SOF/RBV for 12 weeks, and 26 patients with GT-3a, GT-1, or unknown genotype received DCV/SOF for 12 weeks (Figure 1).
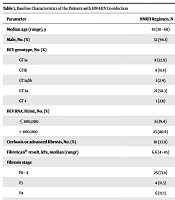
In total, the distribution of HCV genotypes was as follows: 1a (30.2%; 13/43), 1b (11.6%; 5/43), 1a/1b (2.3%; 1/43), 3a (53.5%; 23/43), 4 (2.3%; 1/43), and one was unknown. HIV-1 RNA < 50 copies/mL and CD4 count > 250 cells/mm3 were observed in 81.8% (36/44) and 79.1% (34/43) of patients, respectively. No patients had a platelet count of less than 90,000 per mm3. Also, 5.3% of patients had an albumin level ≤ 3.5 g/dL and 51.2% and 47.6% had the ALT and AST levels of more than 1.5 × ULN, respectively (Table 1).
| Parameter | NNRTI Regimen, N = 36 | PI regimen, N = 8 | Total, N = 44 |
|---|---|---|---|
| Median age (range), y | 41 (30 - 60) | 44 (41 - 62) | 42 (30 - 62) |
| Male, No. (%) | 32 (94.1) | 8 (100.0) | 40 (95.2) |
| HCV genotype, No. (%) | |||
| GT 1a | 8 (22.9) | 5 (62.5) | 13 (30.2) |
| GT1b | 4 (11.4) | 1 (12.5) | 5 (11.6) |
| GT 1a/1b | 1 (2.9) | 0 | 1 (2.3) |
| GT 3a | 21 (58.3) | 2 (25.0) | 23 (53.5) |
| GT 4 | 1 (2.8) | 0 | 1 (2.3) |
| HCV RNA, IU/mL, No. (%) | |||
| ≤ 800,000 | 6 (19.4) | 0 | 6 (15.8) |
| > 800,000 | 25 (80.6) | 7 (100) | 32 (84.2) |
| Cirrhosis or advanced fibrosis, No. (%) | 10 (27.8) | 5 (62.5) | 15 (34.1) |
| FibroScan® result, kPa, median (range) | 6.6 (4 - 45) | 13 (4 - 27) | 7.45 (4 - 45) |
| Fibrosis stage | |||
| F0 – 2 | 25 (71.4) | 3 (37.5) | 28 (65.1) |
| F3 | 4 (11.5) | 1 (12.5) | 5 (11.6) |
| F4 | 6 (17.1) | 4 (50.0) | 10 (23.3) |
| Adding RBV, No. (%) | 10(27.8) | 5(62.5) | 15 (34.1) |
| Treatment duration, No. (%) | |||
| 12 W | 28 (77.8) | 7 (87.5) | 35 (79.5) |
| 24 W | 8 (22.2) | 1 (12.5) | 9 (20.5) |
| HCV-treatment experience, No. (%) | 4 (11.1) | 0 | 4 (9.1) |
| HIV-1 RNA < 50 copies/mL, No. (%) | 30 (83.3) | 6 (75) | 36 (81.8) |
| CD4 count, cells/mm3, median (range) | 432 (67 - 1407) | 412 (140 - 734) | 420 (67 - 1407) |
| Albumin, g/dL, median (range) | 4.4 (3.5 - 5.0) | 4.15 (3.2 - 4.8) | 4.4 (3.2 - 5.0) |
| Albumin ≤ 3.5 g/dL, No. (%) | 1 (3.1) | 1 (16.7) | 2 (5.3) |
| Hemoglobin, g/dL, median (range) | 14.55 (11.6 - 16.0) | 14.5 (11.3 - 16.3) | 14.55 (11.3 - 16.3) |
| Platelets × 103 per mm3, median (range) | 173 (109 - 295) | 157.5 (118 - 320) | 172 (109 - 320) |
| Platelet count ≤ 90,000 per mm3, No. (%) | 0 | 0 | 0 |
| ALT, IU/L, median (range) | 64 (14 - 169) | 39 (21 - 110) | 63 (14 - 169) |
| ALT > 1.5 × ULN, No. (%) | 19 (57.6) | 2 (25) | 21 (51.2) |
| AST, IU/L, median (range) | 58.5 (16 - 170) | 39 (27 - 122) | 53.5 (16 - 170) |
| AST > 1.5 × ULN, No. (%) | 17 (50) | 3 (37.5) | 20 (47.6) |
The highest risk factor for HCV transmission was a history of IV drug use (81.8%, 36/44), followed by using a common syringe (77.3%; 34/44) and tattooing (70.5%; 31/44). There was no statistically significant difference between risk factors and HCV genotypes (Table 2).
| Risk Factors | HCV Genotypes, No. (%) | Total, N = 44 | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| 1a,13 (100) | 1b,5 (100) | 1a/1b,1 (100) | 3a,23 (100) | 4,1 (100) | Unknown, N = 1 | |||
| History of IV addiction | 11 (84.6) | 5 (100) | 1 (100) | 18 (78.3) | 0 | 1 (100) | 36 (81.8) | 0.200 |
| Using a common syringe | 11 (84.6) | 5 (100) | 1 (100) | 16 (69.6) | 0 | 1 (100) | 34 (77.3) | 0.182 |
| Tattooing | 10 (76.9) | 5 (100) | 0 | 15 (65.2) | 0 | 1 (100) | 31 (70.5) | 0.120 |
| Phlebotomy | 7 (53.8) | 3 (60.0) | 0 | 10 (43.5) | 0 | 0 | 20 (45.5) | 0.650 |
| History of blood transfusion | 1 (7.7) | 0 | 0 | 4 (17.4) | 1 (100) | 0 | 6 (13.6) | 0.100 |
| Sexual contacts outside marriage | 8 (61.5) | 4 (80) | 1 (100) | 8 (34.8) | 0 | 0 | 21 (47.7) | 0.158 |
4.2. Outcome and Safety
In both ART-treatment groups (NNRTI regimen and PI regimen), all 44 patients with or without cirrhosis (19 patients with HCV GT-1, 23 with GT-3, and one with GT-4) (100%) completed the HCV treatment course and achieved SVR12 for HCV. Since the rate of SVR12 was a constant, no SVR12-related P-value was computed. Also, 92.6% of patients (25/27) on ART had CD4 count > 250 cells/mm3 at the end of treatment. Median CD4 cell count at the end of treatment was 512 (range; 93 - 1447) cells/mm3 (Table 3).
| Event, n/No. (%) | PI Regimen | NNRTI Regimen | Total |
|---|---|---|---|
| CD4 count > 250 cells/mm3, No. (%) at baseline | 5/7 (71.4) | 29/36 (80.6) | 34/43 (79.1) |
| CD4 count > 250 cells/mm3, No. (%) at end of treatment | 3/4 (75.0) | 22/23 (95.7) | 25/27 (92.6) |
The HCV treatment regimen (DCV/SOF with or without RBV), regardless of concomitant ART, was well-tolerated. Moreover, 15.9% of patients (7/44) experienced AEs, including anorexia, nausea, diarrhea, palpitations, and anxiety. No serious AEs or discontinuation due to AEs were reported. There were no hemoglobin levels < 10 g/dL, and no AST/ALT elevations > 5.0 × ULN at end of treatment (Table 4).
| Event | PI Regimen, N = 8 | NNRTI Regimen, N = 36 | Total, N = 44 |
|---|---|---|---|
| Patients with at least one AE | 3 (37.5) | 4 (11.1) | 7 (15.9) |
| Serious AE | 0 | 0 | 0 |
| AE leading to discontinuation | 0 | 0 | 0 |
| Death | 0 | 0 | 0 |
| Palpitations | 0 | 1 (2.8) | 1 (2.3) |
| Anxiety | 1(12.5) | 0 | 1 (2.3) |
| GI symptoms | |||
| Anorexia | 1 (12.5) | 2 (5.6) | 3 (6.8) |
| Nausea | 1 (12.5) | 0 | 1(2.3) |
| Diarrhea | 0 | 1 (2.8) | 1 (2.3) |
| Platelet count ≤ 50,000 per mm3, No. (%) | 0 | 0 | 0 |
| Hemoglobin level, No. (%) | |||
| < 10 g/dL | 0 | 0 | 0 |
| < 8.5 g/dL | 0 | 0 | 0 |
| ALT, No. (%) | |||
| > 2.5 × ULN | 0 | 3 (10.0) | 3 (8.1) |
| > 5.0 × ULN | 0 | 0 | 0 |
| AST, No. (%) | |||
| > 2.5 × ULN | 0 | 0 | 0 |
| > 5.0 × ULN | 0 | 0 | 0 |
5. Discussion
Approximately 15% - 30% of the HIV-infected patients are co-infected with HCV worldwide (9), and it is shown that the rate of HIV-HCV co-infection is high among people who inject drugs (PWID) (10-12). Similarly, in our study, the highest risk factor was a history of IV drug use (81.8%, 36/44), followed by using a common syringe (77.3%; 34/44) and tattooing (70.5%; 31/44).
In the management of HIV-HCV co-infection, the DCV/SOF ± RBV regimen can be applied for patients with different HCV genotypes, with or without cirrhosis (13). In the present study, all 44 patients (100%) with or without cirrhosis (19 patients with HCV GT-1, 23 with GT-3, and one with GT-4) who received DCV/SOF±RBV achieved SVR12 for HCV. CD4 cell counts remained stable (median CD4 cell count, cells/mm3: 420 at baseline versus 512 at the end of treatment), and HIV control was not compromised by the HCV treatment. Our result was comparable to the data from clinical trials and other real-life studies (8, 10, 12, 14-20), reporting the SVR12 rates of 90% to 100% among HIV-HCV co-infected patients treated with DCV/SOF ± RBV.
Studies showed that SVR rates are lower in patients with treatment experience or cirrhosis compared with non-cirrhotic or treatment-naive individuals (21). Moreover, HCV-GT3 infection accelerates liver fibrosis progression and is associated with the lowest SVR rates with DAAs-based regimens compared with other genotypes, particularly in the presence of cirrhosis (22). In the management of HIV-HCV co-infected patients treated with DCV/SOF, 2015 EASL guidelines recommend either extending treatment duration up to 24 weeks or the addition of RBV for cirrhotic patients with GT-1 or GT-4, and both were adding RBV and 24 weeks of treatment in GT-3, cirrhotic patients (13). In the current study, 5 HCV-GT1 patients with either cirrhosis or advanced fibrosis who treated with DCV/SOF plus RBV for 12 weeks, one HCV GT-4, the cirrhotic patient treated with DCV/SOF plus RBV for 12 weeks, and 9 HCV-GT3 patients with either cirrhosis or advanced fibrosis who received DCV/SOF plus RBV for 24 weeks, achieved SVR12. Our data on GT3-infected patients with either cirrhosis or advanced fibrosis as difficult-to-treat population showed a higher rate of SVR12 than those reported by the phase III ALLY-3C study (23) and Berenguer et al. (15) research, in which after 24 weeks of DCV/SOF/RBV, the SVR12 rate was 93% in 54 HCV-monoinfected, cirrhotic, GT-3 patients and 93.7% in 48 HCV-HIV co-infected, cirrhotic, GT-3 patients, respectively. Nevertheless, our result was similar to that of a study by Rockstroh et al. (10), which showed the SVR12 of 100% in 15 HCV-HIV co-infected, cirrhotic patients with either GT-3 or GT-1 receiving DCV/SOF/RBV for 24 weeks. Similarly, Navarro et al. reported that the SVR12 rate was 100% in 14 HCV-HIV co-infected, cirrhotic, GT-3 patients treated with 24 weeks of DCV/SOF/RBV, and 5 HCV-HIV co-infected, cirrhotic, GT-1 patients treated with 12 weeks of DCV/SOF/RBV (21). Moreover, the SVR rate among our HIV-HCV co-infected patients with GT-3 or GT-1, with cirrhosis or advanced fibrosis who were treated with DCV/SOF/RBV was similar to a report from Mandorfer et al. (19), indicating the SVR rate of 100% in 31 HCV-HIV co-infected patients with either GT-3 or GT-1, cirrhotic or advanced fibrosis who received DCV/SOF without RBV for 24 weeks.
Regarding DAAs, the SVR rates in patients with HIV-HCV co-infection in comparison with those with HCV mono-infection is controversial (6, 14, 24). Our result showed the SVR rates in patients with HIV-HCV co-infection treated with the DCV/SOF ± RBV regimen is similar to those with HCV mono-infection.
In the present study, the DCV/SOF ± RBV regimen was generally well tolerated. No SAEs or treatment discontinuation due to AEs was observed. Also, 15.9% of patients (7/44) experienced AEs, including anorexia, nausea, diarrhea, palpitations, and anxiety. Our result demonstrated a lower rate of AEs than the report from the phase III ALLY-2 Study (8), which showed treatment –related AEs in 72.8% of HIV-HCV co-infected patients treated with 12 weeks of DCV/SOF. The data on common AEs in our study is likely to be underreported.
It seems that the DCV/SOF ± RBV regimen is an optimal treatment strategy for HIV-HCV co-infected patients, especially in situations in which the next generation DDAs are not available or have potential drug-interaction with ART.
In the current study, there were some limitations. First, the small number of patients and subgroups, which limits the definite conclusion on the optimal treatment strategy, especially in HIV-HCV co-infected, cirrhotic patients in the real-world setting. Second, common AEs were likely to be underreported.
In conclusion, our study showed excellent tolerability and efficacy of DCV/SOF ± RBV in Iranian, HIV-HCV co-infected patients with or without cirrhosis. The highest risk factor was a history of IV drug use (81.8%, 36/44), followed by using a common syringe (77.3%; 34/44) and tattooing (70.5%; 31/44). Further research is needed to investigate the optimal treatment strategy for HIV-HCV co-infected patients with decompensated cirrhosis, the regression of liver fibrosis after HCV-treatment in these patient groups, and HCV reinfections after successful DAA treatment in the population at higher risk, such as men who have sex with men (MSM) and PWID.