1. Background
Rheumatoid arthritis (RA) is the most common autoimmune inflammatory disease in the adult population, mainly affecting women aged between 40 - 50 years (1). This condition is marked by symmetrical polyarthritis of small and large joints with consequent synovial involvement and joint degradation. Pain, deformities, and even bone and cartilage destruction may occur as a consequence of progressive damage to the musculoskeletal system (2). Joint abnormalities combined with the systemic impact of the disease, cardiovascular (CV) and pulmonary abnormalities included, may lead to a progressive loss of functional capacity and decreased activities of daily living (ADLs) (3).
Cardiovascular diseases (CVD) are the leading cause of death in individuals with RA and are responsible for up to 50% of deaths (4-7). Individuals with RA undergo cardiovascular events at rates 1.5 - 2 times higher than those of the general population, with an increased risk of myocardial infarction, heart failure, cardiac arrhythmia, cardiac arrest, and sudden death (8, 9). In addition to conventional risk factors, autoimmunity, chronic inflammation, and autonomic dysfunction may play a role in the etiopathogenesis of CVD (10, 11). In RA, all structures of the CV system may be involved and although CVD may be clinically silent, in some cases it can become severe and increase the risk of death (10). Thus, the increased morbidity and mortality justify the diagnosis and early treatment of CVD in the population with RA.
CV autonomic dysfunction is one of the most common RA complications; it occurs in approximately 60% of cases and may precede the development of arthritis (5, 6, 12, 13). Despite the high frequency of CV autonomic dysfunction, studies assessing the autonomic nervous system (ANS) imbalance in individuals with RA are scarce, and the data available are conflicting (5-7, 13). In this context, heart rate variability (HRV) analysis has emerged in recent years as a practical, reproducible, and noninvasive method to assess ANS activity and integrity (4). HRV reduction is an indicator of abnormal and insufficient adaptability of the ANS; it is the expression of an increase in the activity of the sympathetic nervous system and a reduction in the activity of the parasympathetic nervous system, and it is associated with a high risk of CV events (4, 7, 13).
Some studies have discussed connections between the ANS and the autoimmunity associated with RA; thus, it is believed that autoantibodies directed against ANS structures may play an important role in the pathogenesis of autonomic dysfunction (4, 13). CV autonomic dysfunction is also linked to physical inactivity and is more prevalent in people who suffer from chronic pain, as is the case of those with RA (12). HRV is altered in subjects with RA (7); therefore, the assessment of autonomic function using HRV may be useful as part of the CV risk assessment. The utility of HRV assessment can be extended to a timely diagnosis of the altered autonomic function associated with increased mortality in individuals with RA (7).
Although the ANS plays a key role in orchestrating the CV response to stressors, few studies have evaluated HRV during submaximal exercise (14-16), but none of them involved individuals with RA. In this context, it is important to evaluate HRV during submaximal exercise in the RA population to quantify the effects of various interventions on the autonomic modulation of heart rate (HR). Recently, the Glittre-ADL test (GA-T) was proposed; it consists of a set of activities that simulate ADLs, such as arm activities performed without support, rising from a chair, walking, going up and down steps, reaching, handgrip, and carrying weight (17). Thus, assessment of HRV during the performance of the GA-T may be interesting in people with RA because this field test involves tasks that require both the upper and lower limbs, which are affected in individuals with RA. Considering that systemic immunoregulatory impairment and CV autonomic dysfunction are among the main components of RA, we hypothesized that the reduced HRV during exercise may be related to a longer time to perform the GA-T multitasks, physical dysfunction and reduced muscle strength.
2. Objectives
To evaluate autonomic impairment through HRV in women with RA during the GA-T and to correlate these changes with physical functioning and muscle strength.
3. Methods
3.1. Patients
A cross-sectional study was conducted between June 2019 and January 2020 with 20 women (of 27 eligible) with RA aged ≥ 18 years who attend regularly at the Piquet Carneiro Polyclinic of the State University of Rio de Janeiro, Rio de Janeiro, Brazil. The study participants came from a convenience sample. All participants were diagnosed by a rheumatologist using previously proposed RA diagnostic criteria (18). The exclusion criteria were the following: previous diagnosis of hypertension, diabetes mellitus, angina pectoris, myocardial infarction, congestive heart failure, or peripheral neuropathy; any clinically diagnosed RA-related CVD; the use of drugs that could affect autonomic functions, such as antihypertensives and diuretics; prior surgery of the upper limb, hip or lower limb; and joint pain or limitation that could impair GA-T performance using the Clinical Disease Activity Index (CDAI) > 10 points (19). The study was approved by the Research Ethics Committee of the State University of Rio de Janeiro under no. 87594518.4.0000.5259, and all participants signed the informed consent form.
3.2. Clinical Disease Activity Index (CDAI)
All subjects were evaluated by the CDAI, which is calculated from the following four variables: tender joint count, swollen joint count, patient global assessment of disease activity using a visual analog scale, and physician global assessment of disease activity (20). The CDAI varies between 0 - 76 points; higher values indicate higher disease activity (21).
3.3. Health Assessment Questionnaire Disability Index (HAQ-DI)
Subjective measurement of physical functioning was performed using the HAQ-DI. This questionnaire includes questions about the fine movements of the upper limbs and the motor activities of the lower limbs. It includes 20 items divided into 8 categories as follows: dressing, rising, eating, walking, hygiene, reach, grip, and common activities. Respondents give their answers using a scale from 0 (no disability) to 3 (complete disability). The total score is the average score of the eight categories (22).
3.4. Handgrip Strength
Handgrip strength (HGS) was measured using an isometric hydraulic dynamometer (SH5001, Saehan Corporation, Korea) and the hand on the dominant side of the body. The tests followed previous recommendations (23). Participants sat with the elbow flexed at 90° and forearm in the neutral position. Three maximal voluntary contractions were performed at 60-second intervals between tests, and the highest value was used for analysis.
3.5. Quadriceps Strength
Quadriceps strength (QS) was measured using a tensile dynamometer with a sensor capacity of 200 kg (E-lastic 5.0, E-sporte SE, Brazil). Range of motion was determined at 90°, starting at 90° with the knee flexed. Maximum force was assessed after a 5-second sustained contraction in the dominant leg, and the highest value of 3 attempts with 1-minute intervals was considered for analysis (24).
3.6. Glittre ADL Test
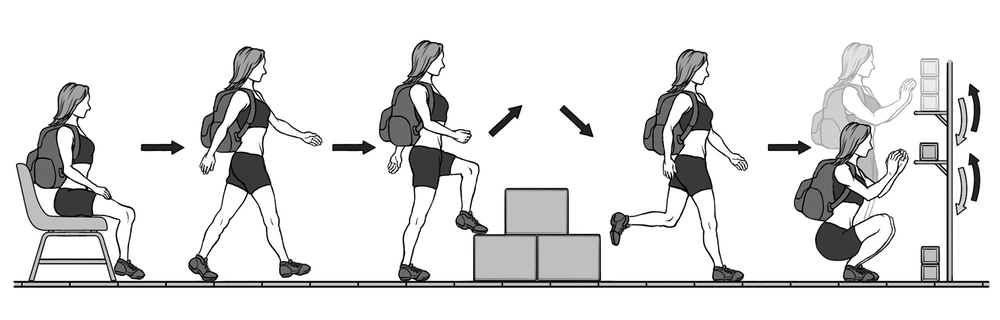
The GA-T (Figure 1) was performed as previously described (17); it consists of carrying a backpack with a weight of 2.5 kg and walking along a 10-m long circuit. According to the GA-T protocol, the participant starts from a sitting position and walks a trajectory that includes a ladder in the middle with two steps up and down. At the end of the circuit, the participant encounters a bookshelf with 3 objects weighing 1 kg each positioned on the highest shelf; the participant is required to move the objects one at a time with both hands from the highest shelf to the lowest one and then to the floor. Then, the objects are replaced on the lowest shelf and then moved to the highest shelf. Then the participant follows the same circuit to return to the starting point. To perform the test, the participant must complete 5 laps in the shortest possible time. The protocol was performed twice at a 30-minute interval, and the shortest time was used for data analysis (25).
3.7. Recording of Autonomic Tone
A telemetric cardiac monitor V800 (Polar Electro Oy, Kempele, Finland) was used to record and analyze the HRV, with its elastic strap fixed to the chest of the participant according to the instructions in the product manual so that it adhered to the skin for correct data collection. The HRV was analyzed throughout the GA-T, from the moment the participant rose from the chair until the end of the last lap. Signals of R-R intervals (iRR) captured by the cardiac monitor were exported to the Polar Flow software (Polar Electro Oy, Kempele, Finland) and then to Excel software. The results were stored in text files and transferred to the Kubios HRV software (version 2.0; Biomedical Signal Analysis Group, Kuopio, Finland) for the calculation of HRV indices through the measurements in the time domain, frequency domain, and nonlinear analysis. The measurements in the time domain analysis were as follows: mean iRR; maximum heart rate (max HR); standard deviation of all iRR (SDNN), which represents global autonomic activity; root mean square of successive differences (RMSSD), which represents vagal modulation; proportion of iRR differing by > 50 ms from previous intervals (pNN50), which also represents vagal modulation; and iRR variability (TINN), which represents global autonomic activity (26). The measurements in the frequency domain analysis were as follows: total power (ms2), which is the power in the HR spectrum between 0.003 - 0.40 Hz and was specified as low frequency [LF, (0.04 - 0.15 Hz)], which is a predominant marker of sympathetic activity, and high frequency [HF, (0.16 - 0.4 Hz)], which is a marker of parasympathetic activity; the LF/HF ratio represents the sympathetic-vagal balance, and a high value for this ratio indicates sympathetic dominance of the cardiac autonomic pulse (26). Finally, we evaluated the following nonlinear measures: standard deviation of the points perpendicular to the line of identity (SD1), which describes the short-term variability (represents parasympathetic modulation); standard deviation along the line of identity (SD2), which describes the long-term variability (represents global cardiac autonomic activity); SD2/SD1 ratio; and approximate entropy (ApEn), which indicates the complexity of the ANS. The recording and analysis were performed as described by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (27).
3.8. Statistical Analysis
Nonparametric methods were applied because none of the variables presented a normal distribution (Gaussian) according to the rejection of the normality hypothesis as assessed by the Shapiro-Wilk test. The results are expressed as median and interquartile ranges or as frequencies (percentages). Comparisons between patients without medication and those using methotrexate or leflunomide in relation to HRV indices were performed using the Mann-Whitney test. The relation between the HRV variables and the other study variables was determined using the Spearman correlation coefficient (rs). Differences and correlations were considered significant when P < 0.05. Data analysis was performed using SAS V. 6.11 software (SAS Institute, Inc., Cary, NC, USA).
4. Results
Among the 27 women with RA who were evaluated for inclusion in the study, 7 were excluded for the following reasons: history of hypertension (n = 2), history of diabetes mellitus (n = 2), previous report of myocardial infarction (n = 1), previous report of congestive heart failure (n = 1), and absence of mobility (n = 1). The median age was 55 (47.5 - 68.8) years, and the median time since diagnosis was 15 (6.50 - 23.5) years. Regarding anthropometric data, the median values for weight, height, and body mass index (BMI) were 70.5 (53.5 - 81.1) kg, 155 (149 - 163) cm, and 26.7 (22.6 - 30.6) kg/m2, respectively. The most commonly used medications were methotrexate (by 14 patients) and leflunomide (by 7 patients). The median value for the CDAI was 6.50 (2 - 9.20) points, while the median value for the HAQ-DI was 0.96 (0.34 - 1.39) points. Regarding muscle strength, the median values for HGS and QS were 18 (8.3 - 20) kgf and 20 (16 - 27.8) kgf, respectively. No participant had a history of smoking. The anthropometric data, physical functioning, and muscle strength are shown in Table 1.
| Variables | Women with Rheumatoid Arthritis |
|---|---|
| Glittre Activities of Daily Living test | |
| Total time, s | 300 (243 - 405) |
| Heart rate variability | |
| HR pre-test, bpm | 85 (74 - 92.3) |
| Max HR, bpm | 118 (112 - 139) |
| Mean iRR, ms | 568 (494 - 595) |
| SDNN, ms | 15.9 (9.23 - 26.7) |
| RMSSD, ms | 24.6 (7.64 - 45.7) |
| pNN50, % | 10.2 (2.30 - 18.5) |
| TINN, ms | 137 (62 - 210) |
| Total power, ms2 | 799 (132 - 1583) |
| LF, ms2 | 89.5 (25 - 148) |
| LF, nu | 71.6 (37.8 - 80.4) |
| HF, ms2 | 52 (16.5 - 193) |
| HF, nu | 28.3 (19.6 - 62.2) |
| LF/HF | 1.21 (0.73 - 1.84) |
| SD1, ms | 18.7 (5.72 - 34.9) |
| SD2, ms | 17.6 (11 - 29.4) |
| SD2/SD1 | 1.18 (0.99 - 2.21) |
| ApEn | 0.89 (0.59 - 1.19) |
Abbreviations: ApEn, approximate entropy; HF, high frequency; HR, heart rate; iRR; R-R intervals; LF, low frequency; pNN50, proportion of iRR differing by > 50 ms from previous intervals; RMSSD, root-mean square of successive differences; SD1, standard deviation of the points perpendicular to the line-of-identity; SD2, standard deviation along the line-of-identity (SD2); SDNN, standard deviation of all iRR; TINN, iRR variability.
aValues expressed as median and interquartile interval values.
The median time required to perform the GA-T tasks was 300 (243 - 405) s, which was 93.2% higher than the expected time to complete it using the predicted Brazilian values for healthy women with the same anthropometric characteristics (25). The greatest difficulties reported by the participants at the end of the GA-T were, in descending order, squatting to perform the bookshelf tasks (n = 11), the ladder tasks (n = 4), the chair tasks (n = 2), the grip tasks (n = 2), and no difficulties (n = 1). The GA-T data and the HRV measurements obtained during the GA-T are shown in Table 1.
The relationship between the HRV measurements, the time to perform the GA-T tasks, the HAQ-DI, the HGS, and the QS are shown in Table 2 and Figure 2. In time domain analysis, the time required to perform the GA-T multitasks showed significant correlations with RMSSD and pNN50; the HAQ-DI showed significant correlation with pNN50. In frequency domain analysis, we observed that the time required to perform the GA-T multitasks significantly correlated with total power, HF and LF/HF, while the HAQ-DI showed significant correlations with total power and HF. In nonlinear measures analysis, the time required to perform the GA-T multitasks showed significant correlations with SD1, SD2/SD1, and ApEn.
| Variables | Total Time, s | HAQ-DI, Points | HGS, kgf | QS, kgf | ||||
|---|---|---|---|---|---|---|---|---|
| rs | P Value | rs | P Value | rs | P Value | rs | P Value | |
| Time domain analysis | ||||||||
| Max HR, bpm | -0.140 | 0.56 | -0.019 | 0.94 | -0.075 | 0.75 | 0.375 | 0.10 |
| Mean iRR, ms | 0.155 | 0.51 | 0.002 | 0.99 | 0.186 | 0.43 | -0.414 | 0.069 |
| SDNN, ms | -0.288 | 0.22 | -0.208 | 0.38 | 0.332 | 0.15 | 0.236 | 0.32 |
| RMSSD, ms | -0.451 | 0.041a | -0.184 | 0.44 | 0.154 | 0.52 | 0.362 | 0.12 |
| pNN50, % | -0.697 | 0.0006a | -0.467 | 0.038a | -0.052 | 0.83 | 0.060 | 0.80 |
| TINN, ms | -0.293 | 0.21 | -0.083 | 0.73 | 0.162 | 0.50 | 0.264 | 0.26 |
| Frequency domain analysis | ||||||||
| Total power, ms2 | -0.683 | 0.0009a | -0.514 | 0.021a | 0.053 | 0.82 | 0.294 | 0.21 |
| LF, ms2 | 0.201 | 0.39 | -0.259 | 0.27 | -0.082 | 0.73 | 0.148 | 0.53 |
| LF, nu | 0.198 | 0.40 | -0.013 | 0.96 | 0.041 | 0.86 | -0.075 | 0.75 |
| HF, ms2 | -0.693 | 0.0007a | -0.444 | 0.049a | 0.034 | 0.89 | 0.325 | 0.16 |
| HF, nu | -0.209 | 0.38 | 0.005 | 0.98 | -0.067 | 0.78 | 0.081 | 0.73 |
| LF/HF | 0.486 | 0.029a | 0.397 | 0.083 | -0.064 | 0.79 | -0.276 | 0.24 |
| Nonlinear measures | ||||||||
| SD1, ms | -0.476 | 0.034a | -0.265 | 0.26 | 0.155 | 0.51 | 0.235 | 0.32 |
| SD2, ms | -0.253 | 0.28 | -0.172 | 0.47 | 0.298 | 0.20 | 0.178 | 0.45 |
| D2/SD1 | 0.498 | 0.025a | 0.259 | 0.27 | -0.035 | 0.88 | -0.307 | 0.19 |
| ApEn | 0.545 | 0.013a | 0.355 | 0.12 | -0.269 | 0.25 | -0.385 | 0.094 |
Abbreviations: ApEn, approximate entropy; HF, high frequency; HR, heart rate; iRR; R-R intervals; LF, low frequency; pNN50, proportion of iRR differing by > 50 ms from previous intervals; RMSSD, root-mean square of successive differences; SD1, standard deviation of the points perpendicular to the line-of-identity; SD2, standard deviation along the line-of-identity (SD2); SDNN, standard deviation of all iRR; TINN, iRR variability.
aSignificant correlations.
We did not observe significant correlation between the HRV indices during the GA-T multitasks and the muscle strength measures (HGS and QS). In addition, we did not observe significant correlation between the HRV indices during the GA-T multitasks and the following variables: time since diagnosis of RA, BMI, and CDAI. When comparing patients without medication to those using methotrexate or leflunomide in relation to HRV indices, no significant difference was found.
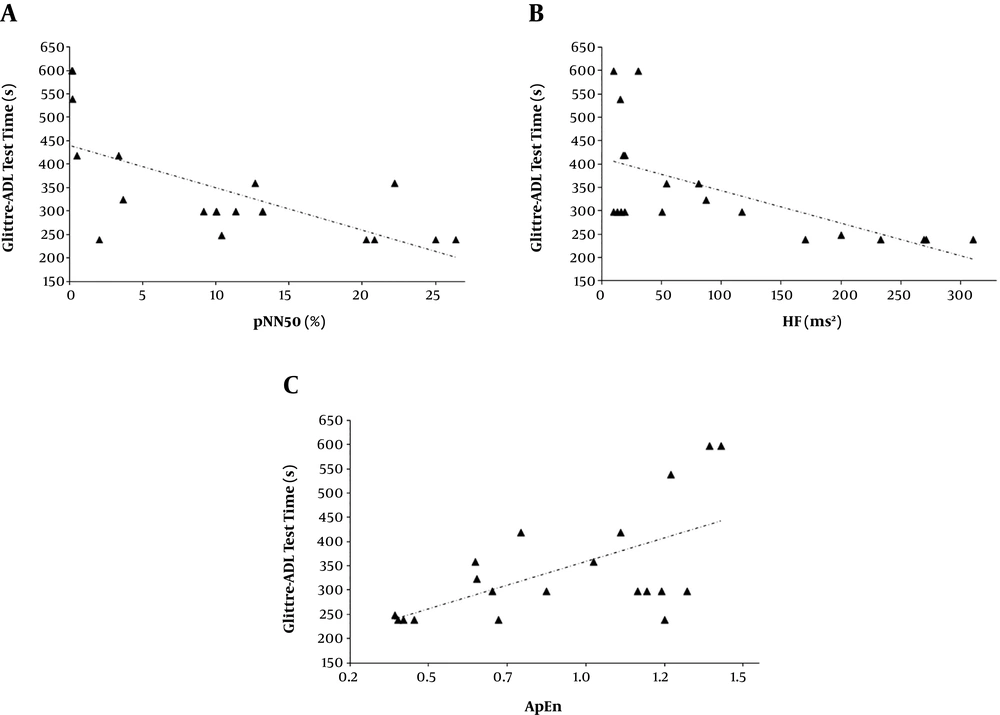
Relationship of the Glittre-ADL test time with the proportion of iRR differing by > 50 ms from previous intervals (pNN50) (rs = -0.697, P = 0.0006) (A), the high frequency in heart rate variability (HF) and the Glittre-ADL test time (rs = -0.693, P = 0.0007) (B), and the approximate entropy (ApEn) and the Glittre-ADL test time (rs = 0.545, P = 0.013) (C).
5. Discussion
The ANS is an important regulatory system that participates in the maintenance of homeostasis. Accurate coordination of the ANS with other organ systems, including the immune system, is of great importance for addressing disturbances of internal and external environments. Considering that immunoregulatory impairment at the local and systemic levels is one of the main components of RA, we evaluated the HRV during GA-T tasks in women with RA. The main findings of the present study were that in women with RA, worse vagal modulation during the GA-T tasks was correlated with a longer time to perform the GA-T. In these women, sympathetic-vagal imbalance and complexity of the ANS were correlated with greater difficulty in performing the tasks of the GA-T. Moreover, there is a relationship between the level of physical functioning and vagal modulation. To our knowledge, this is the first study to evaluate HRV in women with RA during a field test.
Adjustments of the ANS in the heart and blood vessels are necessary to mediate the cardiovascular responses required to meet the metabolic demands of skeletal muscle during exercise (28). We used the GA-T to evaluate sympathetic-vagal behavior during exercise in women with RA; the GA-T is a more complete assessment than the 6-min walk test (6 MWT) for evaluating functional capacity because it better mimics situations of the ADLs and more reliably portrays the burden that patients experience in their daily lives (17). Our sample required almost twice as much time as expected to complete the GA-T, suggesting the multiple manifestations of RA may have had a significant impact on the performance of the test, including cardiovascular and musculoskeletal impacts caused by RA, both of which are often subclinical (1, 2). Although the anti-inflammatory treatments used by individuals with RA -such as corticosteroids and anti-TNF-α therapy- can improve endothelial function by decreasing inflammatory responses and improving HRV, methotrexate can cause endothelial dysfunction due to hyperhomocysteinemia, direct damage to the endothelium, and increased oxidative stress (29). Since 70% of our sample used methotrexate, it is possible that this drug has had an impact on our results.
In the study of autoimmune diseases, premises are focused on how prominent inflammation can spread to impact the CV system. Autonomic dysfunction is a risk factor for CVD, and parasympathetic autonomic dysfunction has been linked to key features of RA, such as inflammation, physical inactivity, and pain (12). In the present study, we observed that during the GA-T, the worse the parasympathetic modulation (represented by the RMSSD, pNN50, HF, and SD1 indices) was, the longer the time required to perform the tasks of the test. In line with our results, a recent meta-analysis showed significantly lower markers of cardiac parasympathetic modulation, measured as RMSSD and HF, in people with RA compared to healthy controls (12). Yadav et al. (7) and Anichkov et al. (30) used a short-term analysis electrocardiogram and 24-hour Holter recordings, respectively, and observed that subjects with RA had significantly lower indices denoting parasympathetic activity compared to healthy controls. This reduction in parasympathetic activity may play a key role in the development of ventricular tachyarrhythmias in RA and may be related to the higher incidence of sudden death in this population. Interestingly, individuals with RA who have relatively high tonus of the vagus nerve -higher parasympathetic parameters measured by HRV- respond better to antirheumatic therapies (5).
Exercise usually increases sympathetic modulation and reduces parasympathetic modulation and overall HRV (31). In this study, sympathetic-vagal imbalance -especially represented by the LF/HF and SD2/SD1 ratios- was associated with greater difficulty performing the GA-T tasks. Consistent with our findings, Bonete et al. (14) evaluated cardiac autonomic control using HRV measurements in patients with type 2 diabetes mellitus during the 6 MWT. In that study, participants with diabetes exhibited lower HRV with a lower parasympathetic impulse, which was represented by the reduction of RMSSD during all phases of the 6 MWT compared to healthy controls. Moreover, the impairment of autonomic control in orthostasis, represented by lower HRV (SDNN and total power), was correlated with lower exercise capacity. Here, it is important to note that while the 6 MWT only incorporates a walking task, the GA-T incorporates multiple tasks that are similar to ADLs. Thus, an important aspect of the present study is that the GA-T combined with HRV analysis may be a useful tool for screening individuals with RA who have an increased risk of CVD. Cumulative evidence indicates that ANS and the immune system are the main contributors to the pathogenesis of CVD in RA (6). It can be argued that over time, worse parasympathetic modulation during exercise may play a role in the pathophysiology of CVD in this population. This observation warrants further investigation.
Autonomic control of the CV system involves an important homeostatic process, which reflects the physiological adaptation that must occur to meet the various metabolic needs imposed on an organism. This adaptive ability of the CV system depends on a complex interaction between its different components and the nervous system. In this sense, an interesting finding in our study was the relationship between the complexity of the ANS, which was evaluated by ApEn, and the time required to perform the GA-T tasks. Large ApEn values indicate high complexity and chaoticity, while lower ApEn values indicate a more regular signal (32). Importantly, ApEn has been reported as a useful measure to predict the individual risk for CVD (33). However, the effects of GA-T activities on the complexity of HRV in people with RA found in the present study are unprecedented and cannot be compared to other studies.
It is increasingly evident that CVD progression depends on the interaction between the ANS and the peripheral target tissues it regulates (34). In addition to measuring HRV, other tests can be used to assess CV autonomic neuropathy, including the measurement of HR and of the blood pressure responses to the standing maneuvers, the Valsalva maneuver, HGS or knee extensions (28, 35). Although we did not measure the HRV during the muscle strength measurement tasks, we did not observe any relationship between the HRV indices and the HGS and QS values. This finding is in contrast to the pathophysiology of the RA, which affects several peripheral joints, with destruction of the affected joints and subsequent impairment of normal function (2); furthermore, a relationship between low HGS and CVD is also well established in the literature (36). Thus, the absence of a relationship between the HRV indices and the HGS and QS values in the present study can be explained at least in part by the fact that we excluded patients with important tender or swollen joints (CDAI > 10), which may have positively impacted in muscle strength measurements. It is also worth mentioning that the sample size is small, which may also have influenced both the estimated effect and statistical significance of the correlation analysis.
Another important point that should be highlighted in our study was the correlations found between the HAQ-DI, which evaluates physical functioning, and the vagal modulation indices obtained during the GA-T. It is possible that the poor vagal modulation in RA may be better evidenced during ADLs, since both the HAQ-DI and GA-T incorporate measurements during ADLs that require activities involving the upper and lower extremities. Finally, it is worth noting that, in line with our findings, Aydemir et al. (35) also found no relationship between autonomic neuropathy and RA duration. This supports the recent evidence that the ANS status triggers the chronic inflammation of RA, and once the process has been triggered, the restoration of the sympathetic-vagal balance becomes independent of the duration of the disease (37).
We must recognize the limitations of the present study. First, the sample size was small, and we did not use a control group; however, we were careful to eliminate confounders that could compromise the results. Second, we evaluated only women, although RA is much more common in women than in men (38). Third, we did not evaluate each of the GA-T activities separately, which could have helped to better explain our findings. Fourth, we did not measure the HRV indices of the participants in a standard maximal graded exercise test to compare with the results of HRV during the GA-T multitasks. Finally, the HRV was the only tool used to evaluate ANS function; thus, the inclusion of tests such as the analysis of blood pressure variability could add information and support the results obtained through HRV analysis. Despite these limitations, our results can serve as a starting point for further studies to assess the integrity of the autonomic control during exercise testing (including GA-T) with a greater number of patients and different degrees of RA activity. Altogether, measuring HRV combined with the GA-T may allow serial evaluations of individuals with RA at different time points, including assessments of therapeutic response, as these two tests are easy to perform and are noninvasive.
5.1. Conclusions
The longer women with RA take to perform the tasks of the GA-T, the worse their parasympathetic modulation, sympathetic-vagal imbalance, and ANS complexity are. Moreover, there is a relationship between the level of physical functioning and vagal modulation.