1. Background
Exercise, without a doubt, has many benefits in the prevention and management of cardiovascular diseases (1). Although regular exercise reduces the risk of cardiovascular diseases, some studies have reported acute coronary syndrome and even sudden cardiac death related to sports activities (2). Some other studies have also reported elevations in cardiac damage biomarkers after prolonged exercise (3-5).
Cardiac troponin (cTn) is the serum cardiac injury biomarker that has been used in the laboratory diagnosis of myocardial infarction (6). Post-exercise cTn elevation without any clinical symptom of myocardial infarction has been previously described (7, 8). Some studies have also reported that the elevation of cTn after exercise was associated with decreased cardiac function and sub-clinical cardiac damage (9, 10). The mechanism of cTn elevation after exercise is still a controversial discussion and is not yet clearly understood. The role of oxidative stress in the mechanism of cardiovascular diseases that involve myocardial infarction has been reported widely as well (11-13). Oxidative stress in cardiac ischemia and reperfusion injury occurs as a result of the accumulation of free radicals or their oxidation products (14). Studies have also reported that oxidative damage happens after prolonged exercise, and the dose relationship has appeared (15, 16).
This study is a continuation of the effort to elucidate the mechanism of cTn elevation after prolonged exercise. In our previous work, we reported a significant elevation of high sensitivity C-reactive protein after long-distance cycling (17) as well as cardiac troponin-I (cTn-I) elevation (18). Our previous report also concluded that post-exercise high cTn-I levels are related to the exercise intensity and inflammatory status, which is represented by neutrophil to lymphocyte ratio (18).
2. Objectives
The current study, therefore, aimed to find out the relationship between the elevation of malondialdehyde (MDA), which is a marker of oxidative stress, and cTn-I after long-distance cycling tours. We hypothesized that MDA elevation is positively associated with elevation of cTn-I after a long-distance cycling tour.
3. Methods
3.1. Design and Participants
We involved male cyclists who were registered to participate in long-distance cycling tours conducted in Indonesia: the 2017 North Coast tour and the 2017 Tour de Borobudur, as described in our previous reports (17, 18). All cyclists had received the invitation to join in this research via a web-based announcement or traditional brochures. Recruitment was stopped as soon as the minimum required sample size was reached. In the beginning, 114 cyclists agreed to participate in the study; however, professional cyclists and individuals with a history of CAD as well as the presence of pathological appearance in the electrocardiograph test were excluded from the study. Consequently, 92 cyclists participated in this study.
Subjects completed the Indonesia North Coast (NC) tour (n = 28) or Tour de Borobudur (TdB) (n = 60) in 2017. NC cyclists rode 240 km with an elevation total of 826 m. The average temperature during riding was 33°C (28°C - 41°C) in sunny weather. TdB cyclists consist of two groups: the first group (TdB 140 K) rode 140 km, with an elevation of 2,754 m, in drizzling and rainy weather, the average temperature during riding was 26°C (21°C - 37°C); the second group (TdB 100 K) rode 100 km in a sunny day, and the average temperature was 30°C (28°C - 34°C). Details of the recruitment are described in a figure as published elsewhere (17, 18).
3.2. Ethics
The committee for ethical research issues of the Medicine Faculty, University of Diponegoro/Dr. Kariadi Hospital in Semarang, Indonesia, approved the research protocol under ID number 607/EC/FK-RSDK/X/2017. Informed consent was acquired from all study subjects. The protocol of the study was recorded at clinicaltrial.gov with ID number NCT03310450. The protocol met the Declaration of Helsinki principles.
3.3. Measurements
Before, during, and after the cycling tours, measurements were conducted as described elsewhere (17, 18), including measurement of the international physical activity questionnaire (IPAQ) (19). Blood specimens were taken before and immediately after cycling and then analyzed in the laboratory. Fractions of whole blood were saved in serum-gel tubes and left for around 45 minutes to clot. Centrifugation was done, then serum was divided into aliquots, frozen, and saved at -80°C for later analysis. All examinations were done using the same setting and calibration in order to reduce dissimilarity. The cTn-I level was quantified using a cTn-I sandwich enzyme-linked immunosorbent assay (ELISA) (Elabscience, E-EL-H0649, USA). The detection limit of the assay was 0.16 ng/dL with a coefficient of variance (CV) of intra-assay precision of 3.78% and inter-assay precision CV of 5.43%. MDA was measured using an MDA competitive ELISA (Elabscience, E-EL-0060, USA). The assay detection limit was 18.75 ng/mL with a detection range of 31.25 - 2000 ng/mL and an intra-assay precision CV < 10 %.
3.4. Statistical Analysis
The distribution of data normality was tested by the Kolmogorov-Smirnov test. All numeric data that were normally distributed were presented as mean ± standard deviation [SD], and the P-value < 0.05 was considered as statistical significance. In the case of the data showing a non-Gaussian distribution, transformation for natural logarithmic was implemented. When the data were still not normally distributed after conducted transformation, statistics for non-parametric were applied, and data were demonstrated as median with interquartile range. Statistical analyses were run using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, version 23.0, IBM Corp., NY, USA).
Paired samples t-test was performed to show the baseline and post-exercise differences in cTn-I and MDA levels. The elevations of cTn-I and MDA were then determined as positive results of the reduction of post-exercise to baseline cTn-I and MDA levels. The determination of parameters that were associated with cTn-I elevation was conducted by bivariate analyses. Analysis of binary logistic regression by the Enter method was done to recognize determinants that were significantly associated with the post-exercise cTn-I elevation. Depending on our postulation and considering confounders, we included body mass index (BMI), systolic blood pressure, and intensity of exercise in our model as probable factors of post-exercise cTn-I elevation. After that, parameters with P-value < 0.10 in bivariate analyses were retained in the eventual model.
4. Results
We involved 92 cyclists in this study, but four cyclists were excluded from analyses since they did not complete the cycling tour and did not complete all requested examinations. The remaining 88 cyclists had an average age of 45.3 ± 11.47 years and an average BMI of 24.2 ± 3.03 kg/m2. With respect to cycling distance, 28 cyclists finished 240 km, 30 covered 140 km, and 30 covered 100 km. Fourteen cyclists failed to report heart rate (HR) data due to human and mechanical error.
The overview of the subjects' characteristics is presented as a table in our previous publication (17, 18). Subjects’ characteristics i.e., age, weight, BMI, IPAQ score, a medical history of hypertension, dyslipidemia, diabetes mellitus, total cholesterol levels, lymphocyte count, monocyte count, and MDA elevation status, were comparable based on the touring category. However, there were significant differences among the tour categories with respect to the family history of CAD, high-density lipoprotein (HDL) cholesterol levels, neutrophil count, resting HR, and exercise intensity. In the North Coast (NC) group, 10.7% cyclists had a family history of CAD, while the Tour de Borobudur 140 km (TdB 140 K) and 100 km (TdB 100 K) groups both had 0%. NC group had higher mean HDL cholesterol level (68.98 ± 19.09 mg/dL) than TdB 140K (52.1 ± 13.9 mg/dL) and TdB 100 K (53.6 ± 12.45 mg/dL) groups. Mean neutrophil count in the NC group was the lowest (50.8 ± 9.03 106/L) compared to 55.7 ± 7.12 106/L in TdB 100 K and 51.8 ± 7.94 106/L in TdB 140 K. NC group had the lowest mean resting HR, i.e., 57.6 ± 8.7 beats per minute (bpm) compared to TdB 140 K (63.2 ± 9.3 bpm) and TdB 100 K (66.7 ± 8.05 bpm). Median exercise intensity in the NC group was the highest (84.3 [75.14 - 90.80] %) compared to 74.5 [45.58 - 90.98] % in TdB 140 K and 80.8 [53.73 - 92.16]% in TdB 100 K groups. Parameters with a significant difference were then considered in the regression analysis as confounders.
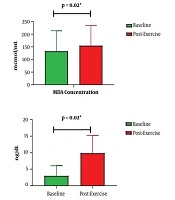
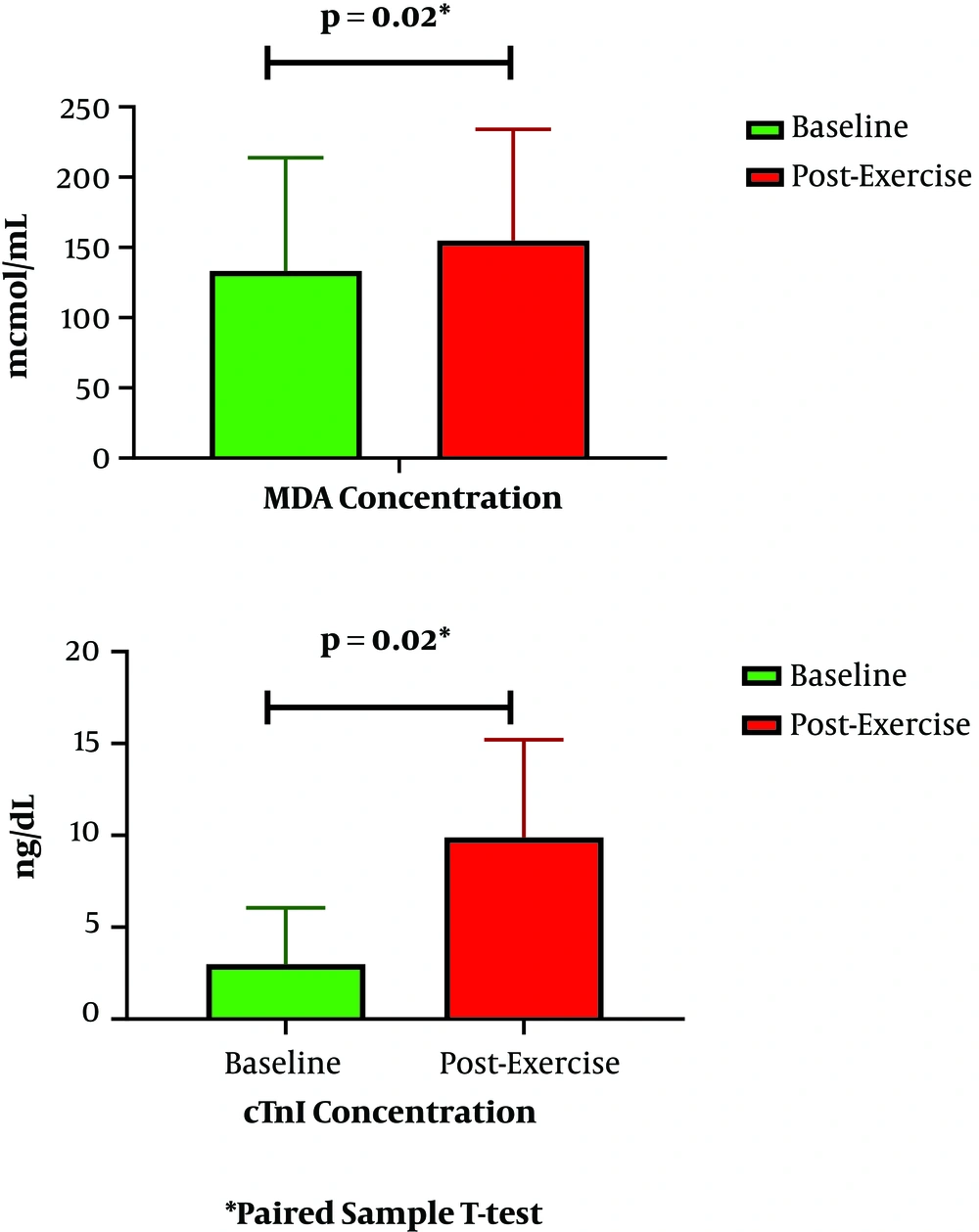
Levels of MDA escalated significantly from 190.18 at baseline to 210.90 µmol/mL post-exercise (P = 0.02) (Figure 1). Similarly, cTn-I levels escalated from 5.16 to 13.65 ng/dL (P = 0.02). Besides this escalation, MDA increase was related to cTn-I increase after cycling (P = 0.04) (Table 1). Based on bivariate analysis, as presented in Tables 1 and 2, other parameters that were associated with cTn-I increase are BMI, systolic blood pressure, tour category, and exercise intensity.
| Variables | cTn-I Elevation | P | |
|---|---|---|---|
| Yes (N = 51) | No (N = 37) | ||
| Age, y, mean ± SD | 45.6 ± 11.45 | 45.0 ± 11.62 | 0.811a |
| BMI, kg/m2, mean ± SD | 24.8 ± 2.96 | 23.3 ± 2.96 | 0.022a |
| Systolic blood pressure, mmHg, mean ± SD | 126 ± 11.2 | 119 ± 14.7 | 0.021a |
| Diastolic blood pressure, mmHg, mean ± SD | 80 ± 6.9 | 81 ± 8.1 | 0.390a |
| Hemoglobin, g/dL, mean ± SD | 14.8 ± 0.9 | 14.8 ± 1.0 | 0.939a |
| IPAQ score, METs, k, mean ± SD | 5.4 ± 4.08 | 4.4 ± 3.59 | 0.893a |
| Medical history, No. (%) | |||
| Hypertension | 10 (19.6) | 3 (8.1) | 1.00b |
| Dyslipidemia | 15 (29.4) | 13 (35.1) | 0.367b |
| Diabetes mellitus | 1 (2.0) | 1 (2.7) | 0.667b |
| CAD in family | 3 (5.9) | 0 (0) | 0.190b |
| Asthma | 5 (9.8) | 3 (8.1) | 0.547b |
| Osteoarthritis | 24 (47.1) | 13 (35.1) | 0.184b |
| Total cholesterol levels, mg/dL, mean ± SD | 214 ± 34.1 | 201 ± 45.2 | 0.135a |
| HDL Cholesterol levels, mg/dL, mean ± SD | 58 ± 17.9 | 57 ± 15.8 | 0,853a |
| Neutrophil, 106/L, mean ± SD | 53 ± 8.5 | 52 ± 7.9 | 0.753a |
| Lymphocyte, 106/L, mean ± SD | 35 ± 8.5 | 36 ± 7.0 | 0.944a |
| Monocyte, 106/L, mean ± SD | 7.4 ± 1.46 | 7.8 ± 1.70 | 0.250a |
| Resting HR, bpm, mean ± SD | 62.7 ± 10.06 | 62.5 ± 8.49 | 0.910a |
| Dehydration, No. (%) | 24; 47.1 | 15; 40.5 | 0.349b |
| MDA elevation, No. (%) | 38; 65.5 | 20; 34.5 | 0.04b |
| Tour category, No. (%) | 0.02b | ||
| NC 240 K | 22; 78.6 | 6; 21.4 | |
| TdB 140 K | 13; 43.3 | 17; 56.7 | |
| TdB 100 K | 16; 53.3 | 4; 46.7 | |
Abbreviations: BMI, body mass index; bpm, beats per minute; CAD, coronary artery disease; cTn-I, cardiac troponin I; HDL, high-density lipoprotein; HR, heart rate; IPAQ, International Physical Activity questionnaires; MDA, malondialdehyde; NC 240 K, 240 km North Coast tour group; SD, standard deviation; TdB 140 K, 140 km Tour de Borobudur group; TdB 100 K: 100 km Tour de Borobudur group.
aIndependent sample t-test.
bChi-square test.
Abbreviations: bpm, beats per minute; cTn-I, cardiac troponin; I HR, heart rate.
aMann-Whitney test.
Parameters with P-value < 0.1, i.e. BMI, systolic blood pressure, MDA elevation, tour category, and exercise intensity, were analyzed in the binary regression logistic model. Binary regression logistic analysis results showed that MDA elevation (odds ratio [OR] = 1.12; 95% confidence interval [CI] = 1.08 - 1.36) and exercise intensity (OR= 1.87; 95% CI = 1.81 - 1.94) were associated with cTn-I elevation after cycling.
5. Discussion
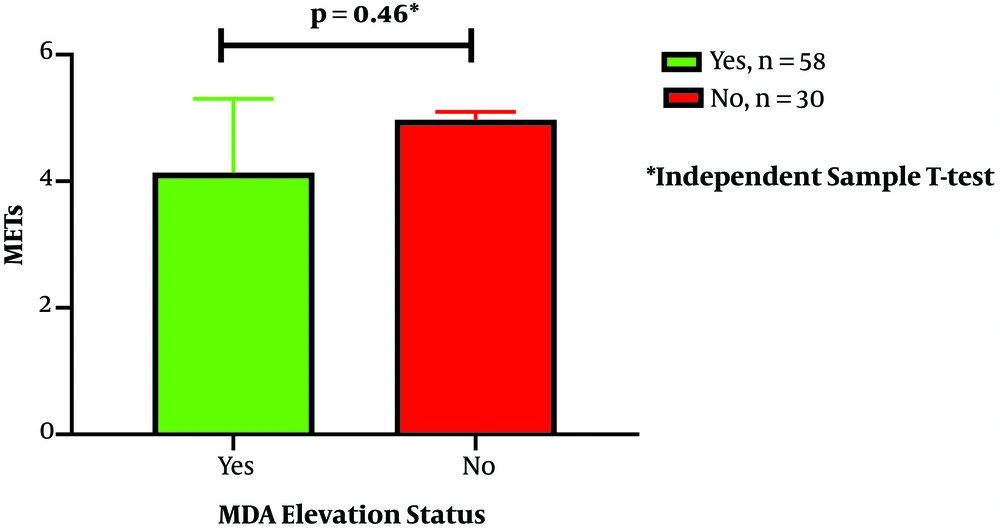
The present study aimed to find out the relationship between the elevation of MDA and cTn-I after long-distance cycling tours. Our findings provide more confirmation that cTn-I elevation after extended exercise is related to oxidative stress, which is determined by MDA elevation. MDA levels increased significantly from baseline to post-exercise measurement. The increase in both parameters can be considered to suggest a synergistic role since there was also a significant association between MDA elevation and cTn-I elevation after a bout of long-distance cycling. Exercise intensity also had a significant association with cTn-I elevation, and together with MDA elevation had 70% of the variant that contributes to cTn-I elevation after exercise as described by binary regression logistic analysis shown in Table 3. Other potential confounders that related to cTn-I elevation in bivariate analysis were BMI, systolic blood pressure, and tour category. The tour category was closely related to exercise intensity because of the difference in cycling distance among the NC 240 K, TdB 140 K, and TdB 100 K groups. There is no difference in the IPAQ score both in the cTnI elevation and MDA elevation status. However, in this study, no sedentary subjects were involved in the study population (Figure 2).
| Number | Parameters | β | OR Adjusted | 95% CI | P |
|---|---|---|---|---|---|
| 1. | MDA elevation | 1.021 | 1.12 | 1.08 - 1.36 | 0.048 |
| 2. | Exercise intensity | 1.137 | 1.87 | 1.81 - 1.94 | 0.001 |
| Constant | 1.137 |
Abbreviations: CI, confidence interval; MDA, malondialdehyde; OR, odds ratio.
aR2 = 0.700 (Nagelkerke).
It is well understood that oxidative stress and inflammatory processes are involved in the pathological mechanism of CAD (20). Oxidative stress also occurs during ischemia/reperfusion as a result of excessive generation of free radicals or their oxidation products. Free radicals induce peroxidation of lipids as well as oxidation of proteins and deoxyribonucleic acid filament breaks (12). Although cTn elevation after exercise is not confirmatory for a cardiac lesion (21), the present study demonstrated the relationship between oxidative stress and the elevation of cTn after cycling. This finding indicates that oxidative stress plays a role in the mechanism of cTn elevation after prolonged cycling. Meanwhile, other studies have demonstrated the elevation of cTn (10) and oxidative stress (22) after prolonged exercise separately.
Regarding the association between oxidative stress and cTn elevation after strenuous exercise, previous studies report significant associations between oxidative stress and cTn elevation after exercise both in human subjects (23) and rat models (24, 25). The previous human study involved a minimum size of homogenous subjects, i.e., nine well-trained marathon runners in a laboratory study (23), while the current study involved 88 heterogeneous participants in long-distance cycling in a field study. Another laboratory study, which used a rat model after forced swimming for 3 hours, elucidates the association between cTn elevation and some conditions, i.e., nitro-oxidative stress, sporadic fragmentation of myocardial structure, and leucocyte infiltration, and functional heart impairment (24).
A previous study, however, had contrary findings, i.e., oxidative stress did not associate with the elevation of cTn after strenuous workouts (16): both oxidative stress and cardiac injury markers, i.e., MDA and cTn-T, were elevated after exercise, as an acute response to exercise; however, the increases were not in synergy (16). The mechanism mediating cTn elevation after exercise is still not clearly understood, but possible mechanisms include excesses of oxidative substrates and preload-induced escalations in stretch-reactive integrins (26), which are in line with the result of the present study. Other possible mechanisms of exercise-induced cTn elevation that have been reported include increasing cardiomyocyte membrane permeability and proteolysis of the cTn complex, leading to efflux of cTn degradation products through the cellular membrane (27), production of cell blebs induced by temporary ischemia (28), excessive cardiomyocyte turnover (29), and myocardial cell necrosis (30).
The present study has several limitations. Specifically, parameters, i.e., MDA and cTn-I, were not measured serially so that the response of these parameters and their association could not be observed with time. A previous study (16) reported that the cTn level was highly elevated immediately after the race and decrease to pre-race level after one to three days after the race. A previous review (31) also reports that exercise-induced oxidative stress involves the production of hydrogen peroxide, hydroxyl radicals, peroxynitrite, superoxide, singlet oxygen, nitric oxide, hyperchlorite, and secondary radical species (31), while the current study did not measure these parameters. Although the present study concluded on the positive association between MDA elevation and cTn-I elevation, exercise-induced oxidative stress can also be produced by the increased energy requirement of contracting skeletal muscles, and other tissue organs such as blood and the lungs, which may also donate to the entire reactive oxygen species during workouts (31). Furthermore, the present study did not involve other parameters that similarly contribute to acute coronary syndrome, i.e. von Willebrand factor, ischemia-modified albumin, and other parameters related to oxidative stress and inflammation.
The present study concluded that elevation of the MDA and exercise intensity are associated with elevation of the cTn-I following long-distance cycling. The present findings add to the confirmation that cTn-I elevation after prolonged exercise is associated with oxidative stress conditions, determined by MDA elevation and exercise intensity. As a recommendation, future studies should involve comprehensive parameters to elucidate exercise-induced cTn elevation mechanisms.