1. Background
Sickle cell disease (SCD) is a hereditary condition with high prevalence and high morbidity and mortality worldwide (1). SCD encompasses a variety of genotypes characterized by the presence of the hemoglobin S gene that can occur in heterozygosity with other abnormal hemoglobin genes. In the homozygous form, the disease is called sickle cell anemia (SCA) and is characterized by a clinically more severe phenotype than the heterozygous form (2). In individuals with SCA, changes in blood rheology, chronic inflammatory processes, and vascular damage play a key role in the occurrence of disease complications (3). From the pathophysiological point of view, this chronic hemolytic condition involves recurrent episodes of inflammation, oxidative stress, and vascular occlusion, causing acute manifestations, especially the vaso-occlusive crisis (VOC), which can also lead to chronic multiple-organ dysfunction, including of the heart (3).
Cardiovascular disease (CVD) is increasingly recognized as a major contributor to premature death in people with SCA (4). The cardiovascular abnormalities in these individuals include cardiomegaly, hyperdynamic precordium, systolic murmurs, and biventricular hypertrophy (5, 6). In addition, individuals with SCA experience VOCs that may occasionally be associated with abnormalities in the cardiac conduction system and myocardial infarction (5). Curiously, sudden death events with no detectable cause at autopsy sums up to 40% of all deaths in adults with SCA (6). However, there is increasing evidence of an abnormal function of the autonomic nervous system (ANS) in SCA, which may lead to an increased risk of sudden death. In addition, ANS dysfunction in SCA seems to be clearly associated with a reduction in the ankle-brachial systolic blood pressure index, erectile dysfunction, syncope, leg ulcers, and acute chest syndrome (ACS) (7).
The ANS imbalance caused by low parasympathetic activity at rest and impaired ANS reactivity during different challenges -both leading to autonomic imbalances- have been reported in individuals with SCA, with the degree of change reflecting the clinical severity (8, 9). The ANS plays an important role in blood flow regulation because the blood vessels, especially the arterioles, are innervated by nerves of the sympathetic nervous system (SNS) (3). Therefore, abnormal autonomic control of peripheral vascular resistance may predispose individuals with SCA to prolonged vasoconstriction in response to stress stimuli and exacerbate ANS changes (4).
The Glittre ADL test (GA-T) -which is an important stress stimulus- was developed to meet the need for a broader and more representative objective evaluation of functionality by involving tasks that simulate activities of daily living (ADLs), including arm activities performed without support, walking, going up stairs, reaching, handgrip and carrying weight (10, 11). Since there is growing interest in the study of heart rate variability (HRV) as it comprises a noninvasive method for assessing the autonomic nerve activity in individuals with SCA, we decided to evaluate HRV during multitasking in the GA-T, which encompasses both upper- and lower-limb activities.
2. Objectives
To evaluate the involvement of the ANS using HRV in adults with SCA during GA-T and to quantify the strength of the correlation of HRV variables with pulmonary and peripheral muscle functions.
3. Methods
3.1. Participants
This cross-sectional study was performed in adults with SCA aged ≥ 18 years who were regularly monitored at Pedro Ernesto University Hospital of the State University of Rio de Janeiro (UERJ), Rio de Janeiro, Brazil. Inclusion criteria for patients comprised a steady state of the disease (i.e. without blood transfusion in the past 3 months) and no occurrence of infection, ACS, or VOC within > 1 month prior to study enrollment (9). The following exclusion criteria were used: known acute or chronic diseases, including CVD, stroke, kidney disease, liver disease, asthma, and diabetes; significant sickle cell symptoms and/or VOC less than 4 weeks from the beginning of the study; use of cardioactive drugs such as β-blockers, glucosides, and antiarrhythmic agents, or other drugs known to affect ANS functions (e.g. antidepressants, diuretics, antihistamines, aspirin); history of surgery on the upper or lower limbs; and inability to walk. We also evaluated 12 healthy controls without sickle cell disease aged ≥ 18 years. This group was recruited at the Augusto Motta University Center, Rio de Janeiro, Brazil and was composed of individuals able to walk who had no previous history of cardiopulmonary or musculoskeletal disease.
The Research Ethics Committee of the State University of Rio de Janeiro approved this study protocol (No. 1.718.917/2016) and all participants signed a written informed consent form.
3.2. Pulmonary Function Testing (PFT)
PFT -spirometry, diffusing capacity for carbon monoxide (DLCO), and respiratory muscle testing (RMT)- were performed on an HDpft 3000 device (nSpire Health, Inc., Longmont, CO, USA) following the standardization and interpretation previously established (12). DLCO was evaluated by the single breath-hold technique and was summarized as the mean of two measures.
The units for the PFTs parameters (absolute values) were as follows: forced vital capacity, L; DLco, mL CO/min/mmHg; maximal inspiratory pressure, cm H2O; and maximal expiratory pressure, cm H2O. However, as recommended by the American Thoracic Society and European Respiratory Society, the interpretation of PFTs requires comparison with predicted values because pulmonary function is influenced by anthropometric (e.g. sex, age and height) and ethnic characteristics to predict the normal pulmonary function (13, 14). The comparison of the absolute values in relation to the predicted values for pulmonary function is intended to separate the effects of pathological conditions from normal variability among healthy subjects. Thus, the predicted values of each participant for spirometry, DLCO, and RMT in the present study were calculated using national equations because of the biotype and ethnic characteristics of our population (15-17).
3.3. Peripheral Muscle Function (PMF)
The PMF was evaluated through handgrip strength (HGS) and quadriceps strength (QS). HGS was measured by a maximal isometric strength test with the SH5001 device (Saehan Corporation, Korea) in the dominant upper limb. The participants were positioned according to standard recommendations (18), with their elbow flexed at 90°, the forearm half-pronated, and the wrist in a neutral position. HGS was summarized as the highest value of three attempts with a 60-s rest time. The QS was evaluated with a tension dynamometer (sensor capacity = 200 kg, E-lastic 5.0, E-sporte SE, Brazil). The range of motion within 90° during the test was determined, starting at 90° flexion at the knee. The maximum force was assessed in the dominant leg after a 5-s sustained isometric contraction. QS was summarized as the highest value from three attempts with a 1-min intervals (19).
3.4. GA-T
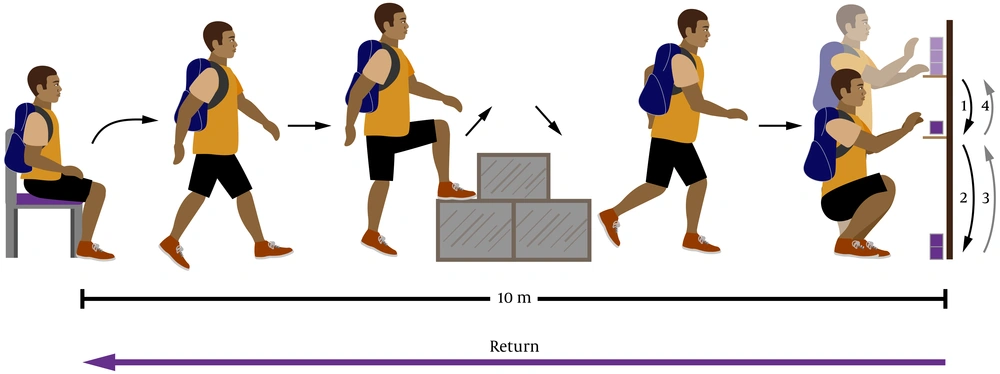
The GA-T was given as previously described (10, 11). The GA-T is a test that comprises multiple tasks that simulate ADLs, including lifting a chair, walking along a path interposed at its midpoint by a staircase, removing boxes from a shelf and placing those boxes on another shelf; and placing the boxes on the floor and then putting them back on a shelf (Figure 1). The protocol was performed twice at an interval of 30 min, and the data for the shorter GA-T were taken for analysis and expressed as total time to complete the multitasks (10, 11).
In the Glittre ADL test, the participant carries a backpack on his back with a weight of 2.5 kg for women and 5 kg for men and walks a 10 m circuit. The participant, from the seated position, walks a course interposed in his half by a box with 2 steps to go up and 2 to go down. After going through the rest of the route, the participant is faced with a shelf containing 3 boxes of 1 kg each, located on the highest shelf, and must then move them, one at a time, to the lowest shelf and, later, up to the floor. Then, the boxes must be replaced on the lowest shelf and later on the highest shelf. Then, the participant comes back, taking the route in reverse. Immediately afterwards, he starts another lap, covering the same circuit, until he completes five laps.
3.5. Autonomic Nervous System Activity
The RR interval (iRR) signals obtained from the electrical cardiac activity and captured by the telemetric cardiac monitor V800 (Polar OY, Finland) were exported to Kubios software (Kuopio, Finland) to calculate the HRV indices during the GA-T. The sampling frequency was 1,000 Hz, and any iRR with a difference > 20% from the previous interval was automatically filtered out by the program (20).
HRV in the time domain, frequency domain, and Poincaré plot non-linear analysis were performed as described by the Task Force recommendations (21-23). Time domain analysis included the following variables: (1) mean iRR; (2) maximum heart rate (maximum HR); (3) standard deviation of all normal iRRs (SDNN), which shows the general ANS activity; (4) root-mean-square difference of successive normal iRRs (RMSSD), showing the parasympathetic nervous system (PNS) modulation; (5) percentage of pairs of consecutive iRRs whose difference is > 50 m (pNN50), which also shows PNS modulation; and (6) triangular interpolation of the iRR histogram (TINN), which shows the general ANS activity (21). The frequency domain measures were mainly total power (TP, 0.04 - 0.15 Hz), which shows the general ANS activity, and its low-frequency component [LF, (0.04 - 0.15 Hz)], which is predominant an indicator of SNS activity, and its high-frequency component [HF, (0.15 - 0.4 Hz)], which is an indicator of PNS activity. Last, the LF/HF ratio shows the sympathovagal balance, a high value indicating SNS dominance (22, 23). At the end, Poincaré plot non-linear measures were evaluated: standard deviation of instantaneous beat-to-beat variability (SD1), which describes short-term variability (shows PNS modulation); standard deviation of long-term continuous iRRs (SD2), which describes long-term variability (shows general ANS activity); the SD2/SD1 ratio; and approximate entropy (ApEn), which detects changes in a time series, indicating ANS complexity.
3.6. Statistical Analysis
The distribution of data was determined by the Shapiro-Wilk test. Data are summarized using mean ± SD, median (interquartile range), or frequency (percentage). Between-group comparisons of demographic data, pulmonary function, PMF, GA-T time, and HRV were evaluated using independent samples t test or the Mann-Whitney test for numerical data and by Fisher’s exact test for categorical data. The correlation between HRV, GA-T time, pulmonary function, and PMF variables were analyzed using the Spearman’s correlation coefficient (rs). The significance level adopted was 5%. Data analysis was performed using SAS 6.11 software (SAS Institute, Inc., Cary, NC, USA).
4. Results
Twenty-three adults with SCA were eligible for the study; seven adults with SCA were excluded because they presented a VOC at less than 4 weeks before the study (n = 3); use of cardioactive drugs or drugs that could affect autonomic functions (n = 3); or inability to walk (n = 1). Thus, the sample evaluated consisted of 16 adults with SCA (with a mean age of 29.9 ± 8 years) and 12 healthy controls without sickle cell disease (with a mean age of 30.5 ± 7.2 years). Compared to healthy controls, adults with SCA showed lower values of pulmonary function (including RMT), HGS, and QS. The demographic data, pulmonary function, and PMF of individuals with SCA and healthy controls are shown in Table 1.
| Variables | Sickle Cell Anemia Group (n = 16) | Control Healthy Group (n = 12) | P Value |
|---|---|---|---|
| Demographic Data | |||
| Age, y | 29.9 ± 8 | 30.5 ± 7.2 | 0.85 |
| Sex, female/male | 9/7 | 8/4 | 0.43 |
| Weight, kg | 63.8 ± 14.4 | 64.9 ± 10.4 | 0.82 |
| Height, cm | 164 ± 7 | 162 ± 5 | 0.43 |
| BMI, kg/m2 | 23.6 ± 4.2 | 24.8 ± 3 | 0.40 |
| Pulmonary Function | |||
| FVC (% predicted) | 75 (58 - 86) | 96 (87 - 100) | 0.0004 |
| DLco (% predicted) | 69 (57 - 98) | 116 (103 - 118) | < 0.0001 |
| PImax (% predicted) | 49 (40 - 63) | 101 (95 - 115) | < 0.0001 |
| PEmax (% predicted) | 48 (25 - 61) | 94 (85 - 108) | < 0.0001 |
| Peripheral Muscle Function | |||
| HGS (kgf) | 24 (17 - 36) | 39 (33 - 46) | 0.006 |
| QS (kgf) | 23 (14 - 31) | 33 (30 - 36) | 0.002 |
Demographic, Pulmonary Function and Peripheral Muscle Function Data of Patients and Healthy Controlsa a
The median time to perform the GA-T tasks was 257 (198 - 368) s in adults with SCA, which was significantly higher than the time observed in the healthy controls. Regarding the HRV variables measured during the GA-T, adults with SCA showed values that differed from the healthy controls for all indices, except for the SD1/SD2 ratio. The most striking differences between the two groups were observed for the following variables: RMSSD, pNN50, HF, LF/HF ratio, SD1, and ApEn (P < 0.0001 for all). The GA-T measures and HRV indexes during the GA-T are shown in Table 2.
| Variables | Sickle Cell Anemia Group (n = 16) | Control Healthy Group (n = 12) | P Value |
|---|---|---|---|
| GA-T | |||
| Total time, s | 257 (198 - 368) | 179 (156 - 195) | 0.007 |
| Heart rate variability | |||
| Maximum HR, bpm | 141 (130 - 166) | 130 (126 - 135) | 0.025 |
| Mean iRR, ms | 499 (426 - 526) | 610 (450 - 765) | 0.020 |
| SDNN, ms | 18 (10 - 23) | 26 (12 - 40) | 0.033 |
| RMSSD, ms | 16 (10 - 19) | 44 (37 - 52) | < 0.0001 |
| pNN50, % | 1.03 (0.35 - 2.53) | 15.9 (12.5 - 22) | < 0.0001 |
| TINN, ms | 124 (100 - 166) | 172 (128 - 203) | 0.031 |
| TP, ms2 | 448 (359 - 550) | 762 (421 - 1365) | 0.008 |
| LF, ms2 | 80 (20 - 96) | 117 (50 - 168) | 0.014 |
| LF, nu | 83 (69 - 87) | 94 (83 - 113) | 0.020 |
| HF, ms2 | 24 (12 - 44) | 295 (199 - 326) | < 0.0001 |
| HF, nu | 15 (12 - 20) | 37 (20 - 52) | 0.003 |
| LF/HF | 1.96 (1.40 - 3.69) | 0.91 (0.69 - 1) | < 0.0001 |
| SD1, ms | 8.20 (6.20 - 12.9) | 36 (28 - 43) | < 0.0001 |
| SD2, ms | 26 (13 - 30) | 50 (17 - 61) | 0.013 |
| SD2/SD1 | 2.64 (2 - 3.42) | 2.51 (1.80 - 3.32) | 0.68 |
| ApEn | 0.72 (0.57 - 0.92) | 1.33 (1.26 - 1.52) | < 0.0001 |
Glittre ADL Test and Heart Rate Variability Data of Patients and Healthy Controlsa a
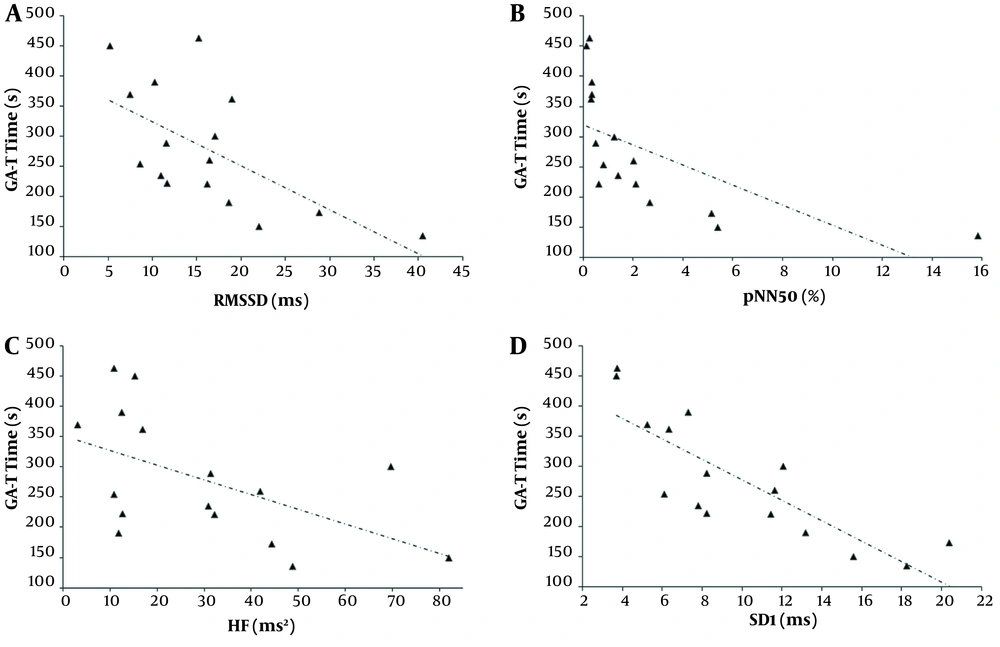
The correlation analysis between HRV variables, GA-T time, pulmonary function, and PMF are shown in Table 3 and Figure 2. The strongest correlations were between the PNS modulation indices (especially RMSSD, pNN50, and SD1) and the GA-T time, DLCO, maximal inspiratory pressure (PImax), HGS, and QS. Additionally, the GA-T time correlated significantly with DLCO (rs = -0.673, P = 0.004), PImax (rs = -0.755, P = 0.0007), HGS (rs = -0.660, P = 0.005), and QS (rs = -0.718, P = 0.002).
| Variables | GA-T time | FVC | DLco | PImax | PEmax | HGS | QS |
|---|---|---|---|---|---|---|---|
| Maximum HR, bpm | 0.100 | -0.171 | -0.303 | 0.113 | -0.006 | -0.215 | -0.192 |
| Mean iRR, ms | -0.274 | 0.229 | 0.477 | 0.118 | 0.105 | 0.339 | 0.336 |
| SDNN, ms | -0.535 a, b | 0.435 | 0.381 | 0.537 a, b | 0.115 | 0.327 | 0.376 |
| RMSSD, ms | -0.650 a, c | 0.271 | 0.605 a, b | 0.620 a, c | 0.302 | 0.503 a, b | 0.508 a, b |
| pNN50, % | -0.932 a, d | 0.188 | 0.578 a, b | 0.733 a, d | 0.245 | 0.501 a, b | 0.538 a, b |
| TINN, ms | -0.526 a, b | 0.186 | 0.383 | 0.627 a, c | 0.075 | 0.120 | 0.191 |
| Total power, ms2 | -0.529 a, b | 0.100 | 0.472 | 0.489 | -0.058 | 0.130 | 0.180 |
| LF, ms2 | -0.074 | 0.415 | 0.277 | 0.258 | 0.128 | -0.007 | -0.085 |
| LF, nu | 0.312 | 0.356 | 0.097 | -0.149 | -0.022 | 0.050 | 0.038 |
| HF, ms2 | -0.579 a, b | 0.138 | 0.518 a, b | 0.396 | -0.010 | 0.224 | 0.299 |
| HF, nu | 0.209 | -0.274 | -0.439 | 0.003 | -0.058 | -0.352 | -0.432 |
| LF/HF | 0.318 | 0.544 a, b | 0.034 | -0.121 | 0.494 | 0.016 | -0.171 |
| SD1, ms | -0.814 a, d | 0.229 | 0.602 a, b | 0.745 a, c | 0.361 | 0.496 a, b | 0.500 a, b |
| SD2, ms | -0.480 | 0.406 | 0.460 | 0.489 | 0.087 | 0.292 | 0.343 |
| SD2/SD1 | 0.215 | 0.397 | 0.184 | -0.141 | -0.173 | -0.088 | -0.093 |
| ApEn | -0.653 a, c | -0.091 | -0.284 | -0.364 | -0.046 | -0.337 | -0.436 |
Spearman's Correlation Coefficients between Heart Rate Variability, Glittre ADL Test, Pulmonary Function, and Peripheral Muscle Strength
Relationships of the Glittre ADL test (GA-T) time with the root-mean-square difference of successive normal iRR (RMSSD, rs = -0.650, P < 0.01), A, the percent of iRR differing by >50 ms from the preceding one (pNN50, rs = -0.932, P < 0.0001); B, the high frequency in heart rate variability (HF, rs = -0.579, P < 0.01); C, and the standard deviation measuring the dispersion of points in the plot perpendicular to the line of identity (SD1, rs = -0.814, P < 0.0001); D, in sickle cell anemia (SCA) individuals.
5. Discussion
The ANS regulates various physiological processes and its physiologic functioning is essential for maintaining stability, even in the presence of stressors. HRV, which is the variability in time and/or frequency of consecutive R waves of the heartbeat, has emerged as a noninvasive electrocardiographic marker of the influence of the activities of the SNS and PNS of the ANS on the sinoatrial node of the heart (24). Assuming the multitasking of the GA-T would be a strong stressor of the SNA in adults with SCA, we evaluated the phenotype of HRV in this population. Our main findings were that, compared to healthy controls, adults with SCA had a marked reduction in HRV during the performance of the GA-T, in regard to the activity of both the SNS and the PNS. This reduced HRV occurred especially at the expense of parasympathetic activity, sympathovagal balance, and abnormal ANS complexity. In these individuals, there was a relationship between a lower HRV (especially of the indices that reflect vagal modulation) and a longer GA-T time, worse pulmonary diffusion, and lower respiratory and peripheral muscle strength. In addition, a worse performance on the GA-T (i.e., a longer time to perform its multiple tasks) was associated with worse pulmonary diffusion and respiratory/peripheral muscle strength. To the best of our knowledge, this study is the first to explore the performance of the ANS during the GA-T in individuals with SCA.
In SCA, the PNS functions seem to be more impaired than the SNS functions (5), with the relative predominance of the action of the SNS promoting peripheral vasoconstriction and reduction of local perfusion and, therefore, increasing the polymerization of hemoglobin S and the sickling of red blood cells (25). In the present study, when individuals with SCA were compared to healthy controls, we observed lower PNS and SNS activity, although there was a clear parasympathetic withdrawal, with significantly lower RMSSD, pNN50, HF, and SD1 (all with P < 0.0001). In addition, we observed a sympathovagal imbalance represented by the high LF/HF ratio. In line with these findings, several studies have shown a persistent and sustained decrease in autonomic fluctuations in people with SCA, with a clear reduction in the PNS activity, both at rest and during exercise (3, 4, 8, 9, 25). Although the exact mechanism of cardiovascular autonomic dysfunction in SCA is not fully clear, many factors contribute to its pathophysiology, including fibromuscular dysplasia, damaged small vessel circulation, focal degeneration and apoptosis, procoagulant activity, abnormal coronary chemoreceptors, and increased oxidative stress (7). In SCA, long-term hypoxemia causes loss of cells in the ambiguous nucleus, which is a set of cells where several vagal efferent axons innervate the ganglionic plexuses on the dorsal surface of both atria (25).
HRV behaves as a complex, non-linear deterministic system with high variability that follows chaos theory and is modulated by the ANS (21). In fact, there is growing interest in evaluating the complexity of short-term cardiovascular control through HRV analysis. Non-linear analyses of the HRV are based on its random and nonperiodic nature and the fact that the HRV dynamics of a healthy heartbeat is variable. Thus, complexity and cardiovascular dynamics are inversely related because they represent less interaction between the ANS regulatory mechanisms (24). In the present study, we observed that adults with SCA had lower ApEn than control adults. In line with our results, data from the literature show that patients with cardiovascular diseases have reduced ANS complexity than healthy individuals, and the reduction in ApEn is an independent predictor of total mortality (26). Given the role of the ANS in people with SCA, it is interesting to assess HRV following the different treatment modalities, including hydroxyurea therapy, blood transfusion and hematopoietic stem cell transplant (27). In this scenario, hydroxyurea therapy for people with SCA reduces the incidence of sudden cardiac death possibly associated with low HRV (28). Using cardiopulmonary exercise testing, a study showed that individuals with SCA on hydroxyurea therapy have heart rate recovery values closer to those seen in healthy controls (29). Considering the potential of HRV analysis during GA-T, we believe that prospective randomized studies are needed to assess ANS changes in response to the treatment of subjects with SCA.
Several things contribute to the poor performance of individuals with SCA during exercise, such as low oxygen-carrying ability of hemoglobin, cardiopulmonary changes, muscle dysfunction, osteoarticular lesions, and poor physical fitness (30). Compared to healthy controls, adults with SCA required a median of almost 50% more time to perform the GA-T tasks. We also noted that the longer the GA-T time was, the lower the HRV was, and PNS activity was especially low. Similar to our findings, Hedreville et al. (8) observed that the PNS activity indices were lower in patients with SCA than in healthy controls when these individuals were subjected to moderate acute exercise. Interestingly, we also observed a significant correlation between longer GA-T time and lower DLCO, which is in agreement with other authors who studied functional exercise capacity in adults with SCA (30). This finding reinforces the use of reduced DLCO as a marker of poor performance during exercise in individuals with SCA. It is also worth noting the relationships observed between the GA-T time and the PMF measures in our study, which indicate that the microvascular obstruction and the oxidative stress characteristic of SCA can negatively impact the peripheral muscles and reduce the performance of individuals during exercise (31-33).
A relationship between ANS behavior and pulmonary function has been discussed under both normal and pathological conditions (32-35). It has been hypothesized that HRV may be influenced by pulmonary function, regardless of cardiac autonomic control, with an increasing-decreasing HR resulting from a biphasic vagal response during the respiratory cycle that leads to instantaneous fluctuations in HRV-the so-called “respiratory sinus arrhythmia” (29, 35). To the best of our knowledge, no previously published work has analyzed the relationship between HRV and pulmonary function in individuals with SCA. Among the pulmonary function indices, we observed more significant correlations between DLCO and vagal modulation parameters. These finding are in line with those observed by Pitocco et al. (32) in individuals with type 1 diabetes, where they found a close relationship between ANS dysfunction and DLCO measures. It is hypothesized that ANS imbalance may modify the functioning of the peripheral and coronary microvasculature and play a role in abnormal regulation of pulmonary microcirculation (33). Thus, we hypothesized that ANS dysfunction is involved in the early reduction in DLCO in people with SCA, possibly due to abnormal blood flow regulation at the pulmonary microvascular level. We also observed a significant relationship between HRV indices (especially those that reflect PNS activity) and PImax. In this sense, it is important to note that the vagus, in addition to being involved in the innervation of the heart's PNS, also plays a role in the innervation of the respiratory muscles; therefore, there is some level of synchronicity between the activities of the respiratory and heart muscles (33).
Autonomic neuropathy may contribute to the pathogenesis of muscle dysfunction in various clinical conditions, with different degrees of impairment of the baroreflex mechanism of skeletal muscle. Here, the lower the HRV (especially the lower the PNS activity) the lower the PMF, as observed by Kabbach et al. (24), Camillo et al. (36), and de Lima et al. (37) in other patient populations, such as patients with chronic obstructive pulmonary disease or terminal liver disease. The relationship between HRV and PMF may have important implications. HGS has been described as a marker of the both regional (upper limbs) and integrity of global function of the individual, whereas an adequate HRV seems to reflect a healthy status with a self-regulated and adaptable cardiovascular system. In addition, HGS has been reported to be negatively associated with all-cause mortality (38). Therefore, an understanding of the relationships between HRV and PMF can help to determine whether HRV is associated with various aspects of SCA, thereby enabling the proposal of intervention strategies that can influence the results.
A major contribution of this study comprises the first evaluation of the phenotype of HRV in adults with SCA as compared to a control group during a submaximal test with multitasking that mimics ADLs. However, we must recognize its main limitations. First, the study population size was small, although the most significant correlations were almost always observed for the same parameters (i.e., PNS modulation indices). Second, we only evaluated adults with SCA, which prevents the generalization of our results to younger age groups of patients with the disease. Finally, the lack of normative values for HRV parameters during the GA-T makes it difficult to establish cutoff points for abnormal HRV in the participants, although we used a control group. However, this study can serve as a theoretical reference for the design of other research aimed at evaluating intervention strategies that consider the ANS during submaximal exercise in patients with SCA. It is also worth noting that the relationships between HRV abnormalities, pulmonary dysfunction, and muscle dysfunction may also contribute to the future research in these individuals.
5.1. Conclusions
Adults with SCA have reduced HRV, and their low PNS activity, sympathovagal imbalance, and the abnormal complexity of the ANS are particularly important. In addition, a lower HRV is associated with a longer time to complete the GA-T, greater impairment in lung diffusion, and greater dysfunction of both respiratory muscle strength and peripheral muscle strength. Thus, the evaluation of HRV during GA-T may be worthwhile for the follow-up of individuals with SCA, including in the evaluation of their response to treatment.