1. Background
The importance of physical activity (PA) and exercise as a part of a healthy lifestyle are well documented. Scientific evidence shows the beneficial effects of PA on global health. PA should be an essential part of the normal development of all children and adolescents. Early in life, especially in infancy and early childhood, PA plays a vital role in a child’s physical, psychological, social, and mental development. The health benefits mentioned from children’s PA include preventing overweightness and obesity, improving skeletal health, increasing heart and lung function, and improving psychological health. The ideal scenario for all young people should be to develop healthy lifestyles at a young age and to continue regular PA in adulthood (1). Nowadays, lack of adequate mobility is one of the problems that threaten the quality of life of children. Given that lack of PA endangers the longevity and quality of life of adults, not engaging children in regular exercise at different ages can significantly affect and exacerbate the prevalence and severity of diseases in adulthood. In this regard, several reports of the World Health Organization have stated the need to increase the level of physical fitness, especially in childhood, adolescence, and youth. Unfortunately, in today’s life, the negative consequences of not paying attention to health have spread, which is one of the essential reasons for not having adequate PA (2).
It is documented that PA with repetition, intensity, and duration and based on regular and scientific programs will have significant health benefits. PA also has significant other additional benefits such as relieving stress and being an excellent mental pastime alongside serious academic work, study, and jobs. Among these merits are: cardiovascular health, increased flexibility, and muscle mass, as well as achieving better bone formation (1). PA as a mechanical stressor can cause biochemical changes (2).
Despite significant scientific advances, the issue of muscle injury remains a severe problem affecting PA. Extroverted contractions in PA are inevitable. When these activities are new to a person, they lead to muscle damage that may last for several days after PA and affect the person’s performance. It is found that muscle damage due to PA leads to decreased contractile function and bruising in skeletal muscle, followed by the development of minor muscle injuries and increased production at the time of contraction. Inflammatory lesions cause more damage. Several factors affect the incidence of muscle injuries in children and adolescents. The characteristics of skeletal muscle depend specifically on the type of muscle fiber. However, changes in the nature and capacity of energy transfer pathways can be independent of muscle fiber. It is well established that metabolic enzymes in skeletal muscle can respond to physiological stimuli such as exercise, as well as disease conditions like muscle wasting, acute airway obstruction, mitochondrial insufficiency, denervation, and eventual disability (3). Serum markers of muscle damage include skeletal, smooth, and cardiac muscle. Muscle tissue is one of the most vascular tissues in the body. Because contraction requires energy, glycogen must reach the muscles. Elevated levels of the enzymes aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), and alanine aminotransferase (ALT) (4, 5).
Markers of musculoskeletal damage include elevated levels of the enzymes AST, LDH, CK, and ALT in the blood (6, 7). The presence of these enzymes in the bloodstream is probably due to mechanical damage to muscle cells and the infiltration of their contents into the interstitial fluid. Lipid membrane peroxidation and subsequent cytolysis appear to be involved in this process (3). In other words, increased levels of these enzymes can be an indicator of cell death and tissue damage after muscle training regardless of proper recycling, which is probably due to increased energy metabolism and muscle tissue damage along with increased protein catabolism in skeletal muscle. However, the level of these enzymes is affected by the duration of exercise, intensity, and type of activity (4, 5, 7).
According to Azidin et al. (2017), the rate of change depends on several factors, including the level of readiness of the subjects, training protocol, nutritional conditions, and other factors, and largely depends on the energy supply path of the activity (aerobic or anaerobic). Although chronic diseases such as obesity, cardiovascular disease, type 2 diabetes, and osteoporosis are commonly identified as adult health problems, they all have their roots in adolescence. The ideal scenario for all adolescents is to develop healthy lifestyles at a young age and to pursue regular PA and adulthood (8-10).
The amount of hemoglobin from childhood to adulthood is increasing, and in contrast, the white blood cells are decreasing. While platelet changes over a lifetime are minimal. On the other hand, hematocrit in boys is increasing throughout childhood and adolescence (2).
Research on the indicators of muscle damage in children has shown that intense PA with extrinsic muscular contractions and high mechanical stress, and with the release of cytokines and the enzymes creatine kinase and lactate dehydrogenase, indicate muscle damage and unexplained changes in endothelial cells. However, in children and adolescents, the response to inflammatory factors, muscle damage, and the relationship between these indicators are less reported. Accordingly, and considering the need to obtain more accurate information about the intensity and duration of sports activities for children and also to evaluate the amount of inflammation and injury caused by intense exercise in these people, this study aimed to investigate the relationship between PA and muscle markers in children and adolescents to determine what level of activities are adequate for children and what level causes harm.
2. Methods
2.1. Study Population
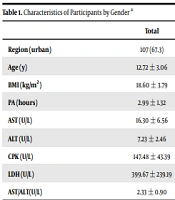
This cross-sectional study was conducted in 2020 with students aged 7 - 18 in Isfahan, Iran. These students were selected using the multi-stage cluster sampling method. From the population, 159 students were selected as the research sample. Students were classified according to the Tanner scale. Based on the tannery, the genital area was examined by observing the pubic hair growth stages and changes in the scrotum and by measuring the length of the testicle in the position where the scrotum was completely stretched. Taking into account the probability of error of the first type equaling 0.05 and power of 90%, and also the correlation coefficient r, the sample size was at least 159. Criteria for inclusion of students aged 7 to 18 years were willingness to participate in the project as well as complete physical health. The exclusion criteria also included reluctance to participate in the project, failure to complete the questionnaire, and having a specific disease. The demographic characteristics and amount of PA of the participants are presented in Table 1.
| Total | Boys (N = 80) | Girls (N = 79) | P Value | |
|---|---|---|---|---|
| Region (urban) | 107 (67.3) | 55 (68.8) | 52 (65.8) | 0.694 b |
| Age (y) | 12.72 ± 3.06 | 12.80 ± 3.10 | 12.64 ± 3.03 | 0.751 |
| BMI (kg/m2) | 18.60 ± 3.79 | 18.89 ± 3.88 | 18.30 ± 3.70 | 0.332 |
| PA (hours) | 2.99 ± 1.32 | 2.99 ± 1.28 | 2.99 ± 1.36 | 0.315 |
| AST (U/L) | 16.30 ± 6.56 | 17.62 ± 6.87 | 14.97 ± 5.98 | 0.011 |
| ALT (U/L) | 7.23 ± 2.46 | 7.41 ± 2.50 | 7.04 ± 2.42 | 0.339 |
| CPK (U/L) | 147.48 ± 43.39 | 157.57 ± 43.10 | 137.38 ± 41.55 | 0.003 |
| LDH (U/L) | 399.67 ± 239.19 | 429.68 ± 244.47 | 367.64 ± 230.77 | 0.109 |
| AST/ALT(U/L) | 2.33 ± 0.90 | 2.46 ± 0.94 | 2.21 ± 0.85 | 0.084 |
Abbreviation: PA, physical activity.
a Values are presented as mean ± SD or No. (%).
b P value was obtained based on tests of chi-squared.
2.2. Procedure and Measurements
2.2.1. Questionnaires
The standard questionnaire of PAQ-C (the physical activity questionnaire for older children) was used to assess the PA level. PAQ-C is a standard 9-item questionnaire to measure the PA level of 8 to 14-year-olds in and out of schools. This questionnaire, by scoring the PA level of students, determines it with a score from 1 to 5. The validity and reliability of the questionnaires have already been evaluated. A panel of experts validated the face validity. Regarding the content validity assessment stage, the questions with a score greater than 0.75 were proven to have appropriate validity. Cronbach’s alpha coefficient for all questionnaires was 0.97, and the Pearson correlation coefficient in the retest stage was 0.94, which confirms the reliability of the questionnaire (11, 12).
2.2.2. Physical Measurements
Weight was also measured with a scale placed on flat ground and the scale closest to 0.1 kg, while participants were wearing light clothing, and height without wearing shoes and the nearest scale being 0.1 cm was measured. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2).
2.2.3. Biochemical Measurements
Eligible students were introduced to the Hashtbehesht laboratory while accompanied by a parent. Then, 6 ml of venous blood samples were collected after 12 hours of overnight fasting. All collection tubes were centrifuged at 3000 - 2500 g*. Immediately after centrifugation, serum samples were evenly distributed in 200 μL tubes and stored at -70°C. Sensitivity of CK with minimum measurement value (3 IU/L), ALT with (4 IU/L), AST with (2 IU/L) and LDH with (5 IU/L) and with the colorimetric method by Pars test kits and using Hitachi 902 biochemistry autoanalyzer was measured.
2.2.4. Statistical Analysis
Continuous variables were expressed as means (standard deviation), and categorical variables as numbers (percentage). The normality of data was assessed using the Kolmogorov-Smirnov test. Comparisons between means of continuous variables in girls and boys were performed using the independent Student t-test. Furthermore, the chi-Square test was used to compare between frequencies of categorical variables in girls and boys. One-way ANOVA was performed to compare mean muscle enzyme levels (including CPK, LDH, ALT, AST, and AST/ALT) in tertiles of PA. Pearson correlation coefficients were used to determine associations between PA with muscle enzyme levels. Linear regression models were performed to investigate the association of adjusted PA with muscle enzyme levels. The PA were adjusted with age, gender, region (urban and rural), and BMI. The analysis data was performed using statistical software STATA 12.0 (STATA Corp, College Station, Texas, USA). P-values less than 0.05 were considered statistically significant.
3. Results
Overall, samples of 159 students were analyzed. The descriptive characteristics of the participants, including demographic characteristics, PA, and muscle enzyme levels by gender, are shown in Table 1. Overall, 50.3% of the students were male. The mean age (standard deviation) was 12.72 (3.06) years. The Kolmogorov-Smirnov test was used to examine how the data were normally distributed. The analysis of the Kolmogorov-Smirnov test showed that the significance level for all research variables is more than 0.05, so the data was normally distributed. Table 2 shows the mean levels of AST, ALT, CPK, LDH, and AST / ALT based on the first, the second, and the third tertiles. Mean AST and CPK were significantly higher in boys than in girls (P ≤ 0.05). The characteristics of the muscle enzyme levels in the PA multiple are shown in Table 2. Participants with higher PA had significantly lower levels of LDH and ALT, respectively. In the third tertile, in students who had higher PA ("3.5" ≤ "PA"), their LDH and ALT levels decreased significantly compared to the first and the second tertile. No significant relationship was found between levels of CPK and PA in the first, the second, and the third tertile.
| T1 (PA < 2.5) | T2 (2.5 ≤ PA < 3.5) | T3 (PA ≥ 3.5) | P-Value | |
|---|---|---|---|---|
| AST | 16.30 ± 7.09 | 16.60 ± 7.50 | 16.07 ± 5.24 | 0.842 |
| ALT | 7.10 ± 2.65 | 7.25 ± 2.61 | 7.32 ± 2.19 | 0.634 |
| CPK | 143.41 ± 39.57 | 162.13 ± 53.08 | 139.39 ± 35.07 | 0.554 |
| LDH | 455.08 ± 268.22 | 417.96 ± 234.87 | 334.86 ± 201.32 | 0.009 |
| AST/ALT | 2.40 ± 1.15 | 2.36 ± 0.87 | 2.25 ± 0.67 | 0.389 |
Abbreviation: PA: physical activity.
a Values are presented as mean ± SD.
Table 3 shows the independent t-test and the mean values of enzymes in the two groups of male and female students based on the PA type. Based on the results, only the level of ALT enzyme in boys at the low and average level of PA showed a significant difference in comparison with girls, without any other significant difference.
| AST | ALT | CPK | LDH | AST - ALT | |
|---|---|---|---|---|---|
| Physical activity | -0.019 | 0.044 | -0.026 | -0.20 a | -0.101 |
a P-value < 0.05.
The Pearson correlation coefficient is presented to analyze the relationship between muscle markers and the PA level. The results obtained in Table 4 indicate that the correlation coefficient between the level of PA and LDH enzyme is -0.20 (P ≤ 0.05). This means that as the PA of these individuals increases, their LDH enzyme levels decrease. On the other hand, based on the results of the Pearson correlation multiplication test, it can be stated that the relationship between the PA level and the CPK enzyme is -0.26 (P > 0.05).
| PA Level | T | P-Value |
|---|---|---|
| LDH | ||
| Low | 0.324 | 0.651 |
| Moderate | 1.78 | 0.061 |
| High | 0.324 | 0.651 |
| CPK | ||
| Low | 0.154 | 0.549 |
| Moderate | 1.254 | 0.074 |
| High | 0.153 | 0.681 |
| AST | ||
| Low | 0.283 | 0.698 |
| Moderate | 1.06 | 0.099 |
| High | 0.861 | 0.107 |
| ALT | ||
| Low | 2.286 | 0.021 |
| Moderate | 3.007 | 0.012 |
| High | 0.907 | 0.846 |
The correlation coefficient was 0.044 between the PA level and ALT enzyme (P = 0.29) and -0.019 with the AST enzyme (P = 0.56).
4. Discussion
This study investigated the relationship between PA levels and muscle markers in 7 - 18-year old students in Isfahan, Iran. The results showed that children and adolescents with higher PA levels had significantly lower LDH and ALT, levels of PA, but this association with muscle enzymes was not significant.
The current findings indicate that there was no significant relationship between the levels of PA and AST enzyme in children and adolescents in the first, second and third tertile. AST is an enzyme that is typically restricted to the cytoplasm of cells and its release into the extracellular environment occurs only with cell death. The results of this study are not consistent with the findings of the studies of Baranco et al. (2018) and Falun (1999) on the increase of the AST enzyme (13, 14). Matsus et al. found that a load-free resistance training session with ten repetitions and one minute of rest did not show any significant increase in the levels of this enzyme (15).
It seems that the type of exercise, recycling time, and intensity of exercise affect the release of these enzymes. Elevated AST enzyme after exercise indicates increased protein catabolism in muscle tissue. In addition, extroverted contraction causes more muscle damage than other types of contraction. Kaynar (2019) showed that increased PA could increase the AST enzyme. It is suggested that the inconsistent findings of studies might be because of differences in the intensity, type, and duration of the training, and the age and gender of the participants (16).
In the present study, children and adolescents with higher PA levels had significantly higher LDH. The cause of LDH secretion is structural changes in muscle tissue following intense activity (17). In fact, the longer the PA of the students, the higher was the LDH level. During exercise, lactate is released from contracting muscles and is consumed by the heart and various oxidative muscles. However, it seems that higher activity in children may not result in intramuscular adaptation, and the nature of strenuous PA may cause inadequate adaptation at all levels, including intramuscular and circulatory, which in turn would result in the activity of enzymes such as increase in LDH, as indicators of muscle and cell damage (13).
Studies confirm that the LDH enzyme, in addition to being active in the process of energy production and lactate, also plays an influential role in treating inflammatory conditions for muscle cells. Wagman et al. (2016) found that by increasing the intensity of exercise and converting activity from aerobic to anaerobic pathway, lactate accumulation increases, and consequently, LDH accumulation will increase. However, some other studies showed contradicting results and an increased in LDH enzyme after exercise. The study of Vakili reported that moderate-intensity aerobic exercise does not increase LDH (18). Barranco (2018) did not observe any change in LDH levels after strenuous physical exercise (13). On the other hand, Kataram et al. (2019) examined the change in blood plasma levels with the intensity of exercise, which showed that low-intensity exercise produces more serotonin, which can improve the body health and also reduce muscle markers in the plasma (19). The reason for the difference between the results of the present study and the study of Kataram et al. (2019) can be related to the difference in the type and duration of the training and also the difference in contractions and muscles involved in the activity (19). The samples in the present study were children and adolescents, but in the study of de Filippi et al. (2012), adults were the sample of the study (20).
The current results did not show any significant relationship between the levels of PA and CPK. Studies show that CPK is the most sensitive enzyme for muscle damage. Studies have shown that the serum concentration of CPK depends on individual characteristics and the type of muscle contractions (21). The results of Gonzalez-Bartholin et al. (2019) and some of the findings of Pettersson et al. (2008) showed a direct relationship between muscle markers and activity level in adults, so that high-intensity exercise increases markers in the blood, however in low- and moderate-intensity exercise, there was no significant change in muscle markers. The results of some studies did not show a significant change in CPK (5, 22).
The results of the present study showed that PA did not cause any significant increase in ALT levels. The results of Kratz et al. (2002) showed that serum ALT increased after PA. This may be because strenuous exercise reduces the blood flow to the liver and kidneys by 5 and 3%, respectively, causing liver damage and increased secretion of these enzymes into the bloodstream (23). Keating and colleagues (2012) showed that aerobic activity did not have a significant effect on reducing the ALT enzyme in people with fatty liver, possibly because of the small sample size without enough power to detect changes in enzymes during exercise accurately. The cellular mechanism of secretion of this enzyme during PA is still unknown, but it is often attributed to structural changes in muscle tissue following intense activity (24). The results of the present study are consistent with other studies that reported that ALT enzyme levels do not increase following short-term PA. Some studies have confirmed the link between muscle damage and the release of muscle enzymes. On the other hand, exercise seems to cause damage to muscle fibers along with rupture of myofibrils and Z lines. Researchers have linked exercise-induced muscle damage to abnormal muscle structure disruption as well as local ischemia (24, 25).
On the other hand, Takahashi et al. (2007) showed that only LDH increases and ALT levels do not change. This may be due to anthropometric characteristics and activity intensity. The rate of change depends on several factors, including readiness of the subjects, training protocol, and nutritional conditions. In general, inflammatory responses are thought to be different in children from adults due to their lower cardiorespiratory fitness in adulthood, so there may be an increase in exercise levels. For children with different levels of physical fitness, in addition to affecting their performance, is associated with risks to cells of other organs, especially muscle cells. Children and adolescents perform PA with different intensities and irregularly since they gradually feel more muscular strength. One of the negative consequences of intense and irregular PA is muscle damage to skeletal muscles (26).
Specific physiological limitations should be considered in the development of exercise programs for children and adolescents because increasing strenuous PA can alter and increase the factors that would impair cellular homeostasis, as shown in the current study. In general, the mechanism and stimulus of injury vary according to the type of activity and perhaps the subject’s condition, which requires further research. Therefore, it is suggested that researchers in future research can study and compare the effect of PA on muscle markers in different people based on age, i.e. children, adolescents, youth, adults, and the elderly. In addition, it is suggested that future research would compare the PA level on muscle injury indices by gender. Different intensities and times on muscle markers should be examined in children and adolescents. This study in the pediatric age group is limited because it is not possible to give the child a high level of fatigue to check for muscle markers. Also, some children get tired after a while and do not want to continue. Finally, equalization of food intake and type of food consumed by the subjects is also considered as a limitation in the present study.
4.1. Conclusions
Overall, the results of this study show that the PA level has a significant relationship with muscle markers in children and adolescents. It is suggested that following the activity of the cellular defense system, it tries to reach a balance. Regular and continuous PA is likely to increase cell defense levels and enzyme activity and might inhibit the activity of free radicals. Increasing PA should be considered in health programs and the daily lifestyle of children and adolescents.