1. Background
Skin infections are relatively common among athletes who compete in sports that are performed on mats, such as gymnastics and wrestling (1). Approximately 20% of wrestling injuries between 1988 and 2004 were due to skin infections. The prevalence of infectious disease in competitive sports that are performed on mat was reported to be 59%. Such infections can be transmitted through skin-to-skin contact with an infected person or through contact with sports mats. Spending long hours in gyms is an important factor in the contamination of these places (2). In general, skin diseases are one of the most important challenges for athletes, in such a way that athletes with obvious skin infections are prohibited from participating in competitions and training sessions (3).
Although personal hygiene plays an important role in ensuring personal health, the role of the environment cannot be ignored. One of the main factors involved in contamination of sports mats is the moisture caused by sweating that provides a suitable environment for the growth and multiplication of bacteria and other pathogens (4). Direct skin-to-skin contact with contaminated surfaces may eventually lead to a variety of skin conditions. Close skin-to-skin contact between athletes and contaminated surfaces such as sports mats increases the risk of infection spread (5). Researchers suggest that playing contact sports leads to a higher risk of staphylococcal skin infections than playing non-contact sports (6). Staphylococcus aureus is a relatively common bacterium that causes a variety of complications, from mild skin problems to infectious dermatitis, sepsis, and toxic shock syndrome. This bacterium is able to settle on the skin or inside the nasal cavity of humans, as about 20% of humans are permanent carriers of this bacterium (7, 8).
It was found that coagulase-negative staphylococci may transfer the infection to other teammates. Among coagulase-negative staphylococci, Staphylococcus epidermidis is the predominant isolate associated with skin infections. An important and key factor in the pathogenesis of this bacterial species is the formation of multilayer biofilms (9). Today, the increased rate of drug resistance among all pathogens has become a serious challenge in the treatment of infectious diseases. Due to a few reports on the antimicrobial effects of biocides and plant extracts on infectious agents isolated from sport mats, development of disinfectants can be a good preventive strategy to combat drug-resistant infections. Chlorhexidine gluconate is a positively-charged biguanide biocide that interacts with the negatively-charged surface of microbial cells, thus destroying the integrity of the cell membrane. Subsequently, this compound penetrates the cell and causes leakage of intracellular components and cell death. Because gram-positive bacteria have a higher negative charge, they are more sensitive to this compound (10).
Medicinal plants have long been used for the treatment of various infections. Compared to antibiotics, alternative antimicrobial agents such as medicinal plant extracts have fewer side effects and exhibit less toxicity. Metabolites in various parts of the pomegranate fruit and tree include sugars, organic acids, alkaloids, polyphenols, flavonoids, anthocyanins, fatty acids, and vitamins. Alkaloids are mostly found in pomegranate peel, and the most important of them are pelletierine, methyl pelletierine, and psuedopelletierine (11).
2. Objectives
The aim of this study was to evaluate and compare the bactericidal activity of chlorhexidine gluconate, and pomegranate peel extract on multi-drug resistant (MDR) S. aureus, and S. epidermidis isolates from sports mats. The results of this study could determine a suitable and effective method for disinfection of sports gyms in order to prevent or minimize the risk of infections.
3. Methods
3.1. Bacterial Isolation
Samples (n = 105) were taken from sports mats in five sports clubs in Gorgan and Kordkoy (Golestan province, Iran) in the spring and summer of 2020. Sample size was determined based on desired accuracy with a confidence level of 95% Source (P = 50%). The ambient temperature of the clubs varied between 25 and 30°C depending on the season. Swab samples were placed in Brain Heart Infusion (BHI) broth and transferred to the laboratory. After 2 hours of incubation at 37°C, they were cultured in mannitol salt agar (Merck, Germany) for 48 hours. Next, staphylococcal species were identified by detecting mannitol-positive colonies, morphology examination, gram staining, and other tests including hemolysis, catalase, coagulase, DNase, and novobiocin susceptibility test.
3.2. Determination of Minimum Inhibitory Concentration (MIC) of the Biocide by the Broth Microdilution Method
In this study, 20% chlorhexidine gluconate biocide (Unilab chemical, India) was used. Based on the protocol of the Clinical and Laboratory Standards Institute (CLSI), the biocide was dissolved in sterile distilled water to prepare a 1000 μg/mL stock solution. A suspension from MDR-S. aureus and S. epidermidis equivalent to a half McFarland standard (3 × 108 CFU/mL) was prepared by reading absorbance 620 nm. The isolates were defined resistant to ciprofloxacin (CIP5), azithromycin (AZM15), aztreonam (ATM30), vancomycin (VA30), and linezolid (LZD10) antibiotic disks (purchased from MAST Co., UK) using Kerby-Bauer method according to the instructions of the Clinical and Laboratory Standards Institute (CLSI, 2015) (12). To determine the minimum inhibitory concenteration (MIC), chlorhexidine gluconate in the range of 1000 to 1 μg/mL was inoculated to a 96-well plate containing Müller Hinton Broth + 2% salt. Finally, the bacterial suspension equivalent to 0.5 McFarland standard was inoculated into the wells. Wells 1 and 12 were considered as negative control (containing MHB and biocide) and positive control (containing MHB and bacterial suspension), respectively. The microplate was incubated for 16 - 20 hours at 37°C. The lowest concentration at which no bacterial growth was observed was reported as the MIC.
3.3. Preparation of Pomegranate Peel Extract
After washing and drying, the pomegranate peel was powdered with an electric mill and mixed in a ratio of 1:10 with distilled water. The mixture was placed on a shaker at 130 rpm for 48 hours. Next, the aqueous extract was obtained by passing the mixture through Whatman® Grade 4 filter paper. The extract was then concentrated using a vacuum rotary evaporator at 60°C. The solvent was then separated, and the resulting extract was dried under vacuum at a temperature of 45°C and pressure of 25 mmHg. After passing the extract through 0.45 micron filters, the extract was stored in a capped, sterile, dark glass container at 4°C until use.
3.4. Determination of Pomegranate Peel Extract MIC
After preparing a stock solution from the extracts with 1% dimethyl sulfoxide, the MIC of pomegranate extract at a range of 1 to 1000 μg/mL was determined by the broth microdilution method. Serial dilutions were made by adding 50λ of the extract to wells of a 96-well plate containing 50λ MHB containing 2% salt. Then, 50λ of MDR-S. aureus and -S. epidermidis suspensions (with turbidity of 0.5 McFarland) were separately inoculated into each well. After overnight incubation at 37°C, the growth rate was measured and compared with that of the positive control (without the extract) and the negative control (without bacterial suspension).
Staphylococcus aureus ATCC 25923 was used as a control strain.
3.5. Statistical Analysis
Data were presented as mean ± standard deviation. All data were analyzed by SPSS software (version 23) using independent t-test, one-way analysis of variance, and Fisher's exact test. All statistical analyses were performed at significance of 0.05.
4. Results
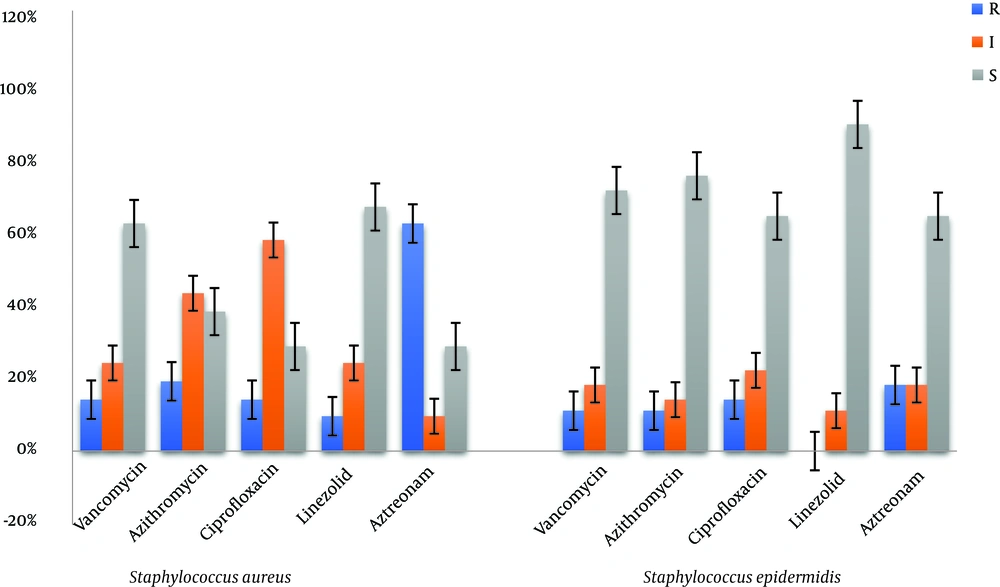
In this Study, the MDR bacteria were based on resistance to glycopeptides, macrolides, quinolones, oxazolidinones, and beta-lactams drug classes. Of 21 (11.4%) S. aureus isolates, 14 (66.7%) were determined as MDR. Of 28 (15.1%) S. epidermidis isolates, 8 (28.6%) were reported as MDR (Figure 1).
4.1. MIC of Biocide and Pomegranate Peel Extract
According to results, chlorhexidine gluconate and pomegranate peel extract inhibited growth of 90% of the MDR S. aureus isolates in a dose-dependent manner and at concentrations of 250 μg/mL and ≥ 500 μg/mL, respectively. The MIC of chlorhexidine gluconate, which inhibited the growth of 90% of MDR S. aureus isolates, was 4-fold lower than the MIC of pomegranate peel extract and 2-fold lower against MDR S. epidermidis isolates (MIC90 ≤ 125 μg/mL). (Tables 1 and 2).
| MDR Isolates | Organism Identification Number | MIC (μg/mL) | |
|---|---|---|---|
| PE | CHG | ||
| S. aureus (n = 14) | SA6 | 500 | 125 |
| SA 22 | 500 | 250 | |
| SA 29 | 1000 | 250 | |
| SA 41 | 500 | 125 | |
| SA 55 | 500 | 250 | |
| SA 62 | 1000 | 125 | |
| SA 88 | 250 | 62.5 | |
| SA 105 | 500 | 125 | |
| SA 110 | 500 | 250 | |
| SA 132 | 1000 | 250 | |
| SA 148 | 1000 | 500 | |
| SA 166 | 1000 | 500 | |
| SA 177 | 500 | 125 | |
| SA 178 | 250 | 62.5 | |
| S. epidermidis (n = 8) | SE 10 | 125 | 31.25 |
| SE 35 | 250 | 31.25 | |
| SE 53 | 500 | 125 | |
| SE 69 | 250 | 15.6 | |
| SE 86 | 250 | 31.25 | |
| SE 108 | 250 | 62.5 | |
| SE 159 | 500 | 250 | |
| SE168 | 250 | 62.5 | |
| Isolate | MIC Range (μg/mL) | P-Value | |
|---|---|---|---|
| Pomegranate Peel Extract | Cholorhexidine Gluconate | ||
| S. aureus | 250 - 1000 | 62.5 - 500 | 0.034a |
| S. epidermidis | 125 - 500 | 31.25 - 250 | 0.073 |
aP < 0/05(aSignificant)
After performing the independence test, it was found that the two criteria of antibiotic classification and severity of effect are related to each other (P-value = 0.041). There was a significant difference (with mean and standard deviation (65.00 ± 0.30) between the minimum concentration of biocides and the minimum concentration of pomegranate extract that inhibits the growth of S. aureus isolates (P = 0.037).
5. Discussion
This study was the first in northern Iran to introduce new disinfectants for sports equipment, especially sports mats, to reduce the possibility of infection transmission during sports activities. During the physical examination of 202 Turkish and Iranian wrestlers, 115 (57%) and 65(20.1%) of participants were observed to have a fungal infection, respectively (13, 14). Since the present study was conducted during the COVID-19 pandemic, the investigated gyms were operating on a part-time basis and in groups of no more than three, which justifies the relatively low frequency of isolates. In general, pathogens and microorganisms can remain on lifeless surfaces for weeks to months; therefore, cleaning and disinfecting surfaces should be performed regularly to reduce risk of infection spread. Skin and soft tissue bacterial infections are characterized by the invasion of bacteria into different layers of the skin, epidermis, dermis, and subcutaneous tissues, which is very common among athletes. These infections are classified into superficial, mild, moderate, and severe. In most cases, skin and soft tissue infections are mild and treated with oral antibiotics. However, about 10% of cases may require hospitalization due to complicated skin and soft tissue infections (15).
In the present study, the frequency of staphylococcal isolates was 20.1%. Previous studies also reported isolation of staphylococci from sports equipment (16, 17). A study in the United States (2014) reported staphylococci as the most commonly isolated bacteria from gyms (18). This difference in the frequency of staphylococcal isolates between the present study and previous studies may be related to geographical factors, sampling location and sampling point, and most importantly, the COVID-19 pandemic. Given the importance of continuous disinfection of stadiums and gyms, it seems essential to seek new and more effective disinfectants. The advantage of using pomegranate peel extract as a natural disinfectant is its compatibility with human health. Contrary to this plant-based extract, chemical disinfectants are harmful to children and people with allergies and can damage the respiratory system and skin in long term (19). The results showed that the pomegranate peel extract had a relatively good antimicrobial effect against both MDR S. aureus and S. epidermidis isolates but had a better inhibitory effect on S. epidermidis at higher concentrations. Another study in Iran showed that the pomegranate peel extract could inhibit growth of at least 50% of the studied bacteria, with the highest inhibitory effect observed against S. aureus (95.84%) (10). In another study in Iran, the antimicrobial effect of pomegranate peel extract was highest against S. aureus (20). Antimicrobial activities of pomegranate have been studied by some researchers. It has been suggested that the inhibitory effect of pomegranate against bacteria is due to presence of ellagitannins (21, 22). Korean researchers proved that ethanolic extract of pomegranate peel had the potential to provide an effective treatment for salmonellosis, so clinical signs and histological damage were rarely observed in the treated mice (23).
In this study, chlorhexidine gluconate had a much better antibacterial effect compared to the pomegranate peel extract. The use of new biocides for the elimination of microbial contamination has become popular. Factors influencing the selection of such compounds include effectiveness of disinfectant, non-toxicity, skin compatibility, cost-effectiveness, odor, availability, and ease of use. Chlorhexidine is an antiseptic that acts against a wide range of gram-positive and gram-negative bacteria, as well as some fungi and viruses (24). An advantage of this antiseptic compound is that it can bind to the surface of many substrates without losing its disinfectant activity and then be released slowly, which results in long-term maintenance of its effectiveness in the environment (25). It has been demonstrated that chlorhexidine gluconate has favorable antimicrobial activity against oral bacteria (26). Moreover, MIC concentrations of chlorhexidine gluconate could prevent biofilm formation in bacteria causing nosocomial infections, while sub-MIC concentrations of this compound can stimulate biofilm production (27).
A previous study reported that both chlorhexidine and chamomile extract had a good inhibitory activity against S. aureus and Streptococcus pneumoniae isolates from patients in the intensive care unit, but the antimicrobial effect of chlorhexidine was generally greater than that of chamomile extract (28). Similarly, in our study, chlorhexidine gluconate had stronger antibacterial properties than the pomegranate peel extract. The combination of such disinfectants with plant extracts may also increase their antimicrobial effects, as some researchers have confirmed the synergistic effects of various factors in reducing the growth of bacteria in the environment (29).
5.1. Conclusions
The results indicate that the use of chlorhexidine in appropriate concentrations can prevent the growth of coagulase-positive and coagulase-negative staphylococci. Given the increasing prevalence of MDR strains, further research is needed to verify the efficacy of this biocide for the disinfection of sports clubs and gyms.