1. Background
Metabolic disorders lead to the development of chronic diseases such as cardiovascular diseases and type 2 diabetes (T2D) (1). Type 2 diabetes is characterized by hyperglycemia, fasting hyperinsulinemia, and insulin resistance (2). The World Health Organization estimates that by 2030, the number of T2D patients will be approximately 360 million worldwide (3). It has been shown that the complications of diabetes are more severe in females than males (4). Females with T2D have a higher risk for vascular disease (4), coronary heart disease (5), and stroke (6) compared with males. Moreover, a history of gestational diabetes or polycystic ovary syndrome can increase the risk of developing T2D in females (7, 8).
Type 2 diabetes is associated with endothelial dysfunction (9). Early detection of endothelial dysfunction in T2D can prevent atherosclerotic processes and vascular complications (10). The endothelial dysfunction is related to the impairment of shear stress and NO release in arteries, which can be detected via a non-invasive technique named flow-mediated dilatation (FMD) (11, 12). It has been shown that a reduced level of NO can lead to the reduction of FMD in T2D patients (11, 12). Nitric oxide is the most potent vasodilator synthesized in the endothelium by the enzyme nitric oxide synthase (eNOS) and has an important role in endothelial function (13). Some studies show that NO bioavailability (14) and serum level of adropin (15, 16) are low in people with T2D, and both of them are correlated to coronary atherosclerosis (15, 17, 18). Adropin is a relatively new biomarker expressed in vascular endothelial cells and would affect endothelial function (19). Adropin can induce up-regulation of eNOS and promote NO levels (19). Accordingly, a low level of serum adropin is related to the reduced FMD in T2D patients (10).
It is proved that lifestyle modification, such as exercise training, can improve vascular structure and function in people with T2D (20-22). There are also suggestions that exercise training improves FMD (23) and increases adropin and NO levels (24, 25).
High-intensity interval training (HIIT) has been described as alternating periods of relatively intense work and passive or active mild recovery (26). This type of training can be a time-efficient exercise option for exercise adherence for those who cite lack of time as a barrier to regular exercise. Furthermore, emerging research suggests that HIIT can provide physiological benefits for patients with a chronic disease (27). Interestingly, a study in patients with T2D showed that HIIT led to more significant FMD improvement compared to low-intensity exercise training (28). In addition, it is reported that HIIT has superior cardiovascular effects than moderate continuous exercises in heart failure patients, regarding shear stress and NO bioavailability (29). It is accepted that exercise training can improve endothelial function; however, there is little evidence on the effect of HIIT on FMD and circulating levels of NO in females with T2D. Moreover, it is unclear whether the serum adropin levels will elevate in T2D patients after HIIT.
2. Objectives
Therefore, this study aimed to investigate the effects of a 12-week HITT program on the circulating levels of adropin, NO, and FMD as indicators of endothelial function in females with T2D.
3. Methods
3.1. Participants and Study Design
Participants of this study were 30 diabetic females recruited from the diabetes clinic of [edited out for blind review] by convenience sampling method. Inclusion criteria consisted of volunteer females, aged between 20 to 44 years, with at least two years history of T2D according to the American Diabetes Association criteria as follows: (1) HbA1c ≥ 6.5%; and (2) plasma glucose ≥ 7 mmol/L after an overnight fast. Exclusion criteria were any exercise training program from at least six months before the beginning of the study, fasting blood glucose ≥ 22 mmol/L, HbA1c ≥ 10%, myocardial infarction, coronary artery bypass surgery or angioplasty, chronic heart failure, cardiac arrhythmia, pulmonary disorder, autoimmune disorder, insulin therapy, functional limitations, liver or kidney disease. The trial was approved by the Ethics Committee of the (edited out for blind review) University and conducted according to the declaration of Helsinki. All participants provided written informed consent before participating in the study.
Anthropometric indices of the participants such as height, body mass, waist and hip circumference, and subcutaneous fat thickness were measured a week before the intervention. Then, participants were randomly assigned into two equal groups of HIIT and control using permuted block randomization. Blood sampling was performed two days before and three days after the intervention, as pre- and post-test. All participants were encouraged to maintain their food intake as usual during the experiment. Besides, in order to hinder the possible dietary effects on the blood variables, a 24-hour dietary recall was administered before the pre-test as the reference value for the post-test.
Participants in the control group were asked to maintain the activities of daily living without any training program during the study period. Correspondingly, participants of both groups continued their prior regimen with metformin under their medical supervision. Additionally, participants of the HIIT group performed a high-intensity interval-training program three times a week for 12 weeks.
3.2. Exercise Training Intervention
The exercise training consisted of 10-min of warm-up, including walking, running, and stretching, followed by four 4-min running intervals at 85 - 95% of HRmax separated by 3-min intervals at 50 - 60% of HRmax, and ended with a 5-min cool-down at 40% of HRmax (30). The running exercise was performed on a treadmill (Landice, Randolph, NJ, USA). Participants wore a heart rate monitor (Polar Beat, Polar Electro, Kempele, Finland), to ensure the appropriate intensity during exercise sessions.
3.3. Measurements
According to American College of Cardiology guidelines, brachial artery FMD was assessed three days before and two days after the intervention using a high-resolution Doppler ultrasound system (AtCor Medical, Solingen, Germany). All participants were asked to fast and avoid any exercises, caffeine, alcohol, drugs, stimulants, and medications for at least eight hours before the measurement (31). Measurement was obtained from the left arm at the angle of 80° with the cuff placed distal to the olecranon process. Baseline brachial artery diameter was measured before cuff inflation for a period of at least one minute. Then the cuff around the left forearm was inflated rapidly to 50 mmHg above systolic blood pressure for five minutes to induce forearm ischemia and the subsequent hyperemic stimulus. Three minutes after deflating the cuff, the second measurement of brachial artery diameter was performed. The FMD was expressed as the change in artery diameter using the equation (32):
Venous blood samples (5 mL) were collected two days before and three days after the intervention, after overnight fasting. The samples were drawn into a lithium heparin tube and promptly centrifuged at 2000 g for 15 min at 4°C, and plasma and serum were stored at −80°C for subsequent analysis. Biochemical indices, including FBS and lipid profile, were assessed with colorimetric enzyme reactions in an automatic chemical analyzer (Cobas C111; Roche Diagnostics, Indianapolis, IN, USA). HbA1c was measured by anion exchange chromatography, and plasma insulin was determined with an ELISA method (Mercodia, Uppsala, Sweden). Serum adropin and plasma NO concentrations were measured using an ELISA (LifeSpan BioSciences, Inc., Seattle, WA, USA) and Griess (R&D System Europe, Ltd, Abingdon, UK) methods, according to the manufacturer's instructions. The insulin resistance levels were calculated by the formula of the homeostasis model assessment:
Anthropometric characteristics were assessed before the FMD measurements at pre- and post-test. The body fat percentage was calculated using the Jackson-Pollock 3-site skinfold formula (suprailiac, thigh, and triceps) by a Lange skinfold caliper (Beta Technology, Santa Cruz, CA). WHR was calculated by dividing waist by hip circumference.
3.4. Statistical Analysis
All data are reported as mean ± SD. The Shapiro-Wilk test was used to verify the normal distributions of the data. The ANOVA with repeated measurements was applied to detect the effect of time (pre vs. post), group (control vs. HIIT), and interaction (time × group). In the case of significant time or interaction effect, paired t-test was used to assess within-group differences. For the HOMA-IR, in which normality was not confirmed, the Mann-Whitney U test and the Wilcoxon signed-rank test were used to assess between and within group differences, respectively. A level of P < 0.05 was considered to be statistically significant. Statistical analyzes were performed using SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA).
4. Results
The ANOVA with repeated measurements showed significant time [F (1, 28) = 18.120; P < 0.001] and interaction [F (1, 28) = 17.490; P < 0.001] effects for FMD%. The paired t-test test revealed a significant increase in the HIIT (P = 0.001), but not in the control group (Figure 1).
There were also significant time [F (1, 28) = 4.812; P = 0.037] and interaction [F (1, 28) = 9.409; P = 0.005] effects for the adropin levels. Within groups comparisons showed significant increase in the HIIT group [t (14) = -2.889; P = 0.012], but no significant changes in the control group [t (14) = 1.056; P = 0.309] (Figure 1).
The NO levels also increased significantly as a result of the intervention. As it was shown with significant time [F (1, 28) = 21.045; P < 0.001] and interaction effects [F (1, 28) = 30.982; P < 0.001]; as well as, within the HIIT group increase [t (14) = -5.590; P < 0.001], and no significant change in the control group [t (14) = 1.169; P = 0.262] (Figure 1).
For the HOMA-IR, non-parametric tests showed a non-significant increase from 4.23 ± 1.6 to 4.09 ± 1.6 in control (Z = -0.852, P = 0.394), and a significant decrease from 4.31 ± 1.7 to 2.54 ± 0.9 in the HIIT group (Z = -3.408, P = 0.001). The levels of HOMA-IR at post-test were also significantly different between the groups (Z = -2.883, P = 0.004).
There were also significant positive effects of the intervention on the lipid profile (except HDL), FBS, and HbA1c, and anthropometric variables. The statistical reports of these variables are summarized in Table 1.
| Variables | Control Group | HIIT Group | Interaction Effect (P-Value) | Time Effect (P-Value) | ||
|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | |||
| Weight (kg) | 76.07 ± 7.7 | 76.80 ± 7.1 | 77.93 ± 9.5 | 74.27 ± 9.0 a | 0.000 | 0.000 |
| BMI (kg.m-2) | 28.70 ± 1.5 | 29.00 ± 1.5 | 29.20 ± 1.3 | 27.85 ± 1.2 a | 0.000 | 0.000 |
| Body fat (%) | 33.04 ± 3.6 | 33.31 ± 3.7 | 33.53 ± 3.5 | 29.94 ± 1.9 a | 0.000 | 0.000 |
| WHR | 0.84 ± 0.07 | 0.85 ± 0.07 | 0.87 ± .11 | 0.78 ± 0.09 a | 0.000 | 0.000 |
| Cholesterol (mmol.L-1) | 4.95 ± 1.1 | 5.11 ± 1.1** | 5.07 ± 1.1 | 4.47 ± 0.9 a | 0.000 | 0.000 |
| Triglycerides (mmol.L-1) | 1.77 ± 0.4 | 1.82 ± 0.3 | 1.74 ± 0.3 | 1.29 ± 0.3 a | 0.017 | 0.003 |
| HDL (mmol.L-1) | 1.22 ± 0.2 | 1.09 ± 0.2 | 1.18 ± 0.3 | 1.37 ± 0.3 | 0.580 | 0.014 |
| LDL (mmol.L-1) | 3.29 ± 1.0 | 3.27 ± 1.0 | 3.45 ± 1.1 | 2.73 ± 0.9 b | 0.025 | 0.036 |
| FBS (mmol.L-1) | 10.79 ± 2.2 | 10.42 ± 2.2 | 10.89 ± 2.4 | 8.40 ± 1.7 a | 0.000 | 0.000 |
| HbA1c (%) | 8.00 ± 0.8 | 8.18 ± 0.9 | 7.82 ± 1.0 | 6.88 ± 0.7 a | 0.003 | 0.000 |
Abbreviation: WHR, waist to hip ratio.
a P < 0.01 vs. pre-test (paired t-test).
b P < 0.05 vs. pre-test.
5. Discussion
The main finding of the present study is that 12 weeks of HITT effectively improved endothelial function markers in females with T2D. This improvement involved an increase in the FMD of the brachial artery, serum adropin, and plasma NO levels. The levels of FBS, HbA1c, and insulin resistance were significantly reduced in the HITT group. Our results are consistent with previous studies regarding the efficiency of the interval training programs on glycemic control in people with T2D (33, 34). The improved glycemic control can be related to glucose uptake, glucose transporter protein levels, and local factors such as calcium and mechanical factors (33). Furthermore, exercise training intensity is an essential factor in improving glycemic control, the release of local factors, and strengthening the mechanical factors (35). Therefore, HIIT appears to be an effective protocol to improve glycemic control in females with T2D.
Research in HIIT interventions to assess endothelial function in T2D patients is limited. Mitranun et al. (2014) indicated that FMD increased following 12 weeks of the HIIT program (4 – 6 × 1-min intervals at 80 - 85% VO2peak, separated by 4 min active recovery at 50 - 60% VO2peak) in patients with T2D (33). Madsen et al. (2015) reported a 23% improvement of FMD after low volume HIIT (10 × 1 min intervals each interspersed by 1 min of active recovery for 12 weeks) in T2D patients (20). In another study, Ghardashi et al. (2018) showed that 12 weeks of HIIT lead to increased FMD in type 2 diabetes patients (34). In the present study, we observed a 68% increase in FMD after the HIIT protocol. A review article by Ashor et al. showed that every percent of exercise-induced-increase in the FMD could decrease the risk of cardiovascular events by 13% (36). Adequate blood flow and shear stress could be considered as a mechanism to improve FMD. Exercise-induced increase in shear stress results in more activation of potassium channels, which causes an influx of calcium into the endothelial cells. Increased intracellular calcium would lead to activation of eNOS, elevation in NO production, and ultimately increased FMD (34, 37, 38). Our finding in the present study showed that NO increased significantly after HIIT intervention in T2D females. The increase in NO may contribute to improving FMD that we recorded in the brachial artery in T2D females.
Previous studies have shown that low adropin level is a risk factor for hypertension (39), coronary artery disease (17), and metabolic syndrome (40). To our knowledge, the present study is the first to investigate the effect of exercise training on the adropin levels in T2D patients. Although, two recent studies have reported that aerobic exercise can increase circulating adropin levels in obese elderly adults (25) and in middle-aged and older adults (24). Our study found that 12 weeks of HIIT intervention significantly increased the serum level of adropin in T2D females. This elevation in serum adropin could be considered as an index of endothelial function improvement. However, the mechanism of the increase in serum adropin levels induced by exercise training is unclear. An in vitro study showed that adropin treatment raised eNOS mRNA expression, which results in increased NO production (19). Adropin-induced eNOS activation is mediated by the activating phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway in endothelial cells, resulting in increased NO production (19). Furthermore, this study showed that the lipid profile improved after the HIIT intervention. A mouse study revealed an inverse relationship between plasma adropin concentration and lipid profile (41). Therefore, adropin levels may provide a useful biomarker for identifying dyslipidemia.
The lack of dietary control during the intervention could be mentioned as a limitation for the present study. To minimize the influence of diet, we continually reminded subjects of their commitment to maintaining their current dietary habits and asked them to refrain from taking a high-nitrate diet, caffeine, and alcohol for 48 hours before the blood sampling. In addition, we used a 24-hour dietary recall before the pre-test as the reference value for the post-test.
5.1. Conclusions
The results of this study showed that 12 weeks of HIIT, including three sessions a week running at 85 - 95% of HRmax, involving four 4-minute intervals with 3-minute active rests, could improve the endothelial function in females with T2D, as is demonstrated by the increase in FMD%, adropin and NO. In addition, these patients can use this short duration training protocol to manage the glucose-related indices, including FBS, HbA1c, and insulin resistance; as well as the lipid profile and the body composition variables.
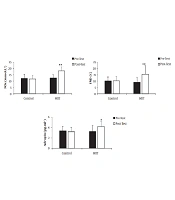
![FMD%, Adropin, and NOx levels, before and after the intervention in both groups. Data are expressed as means ± SD [** P < 0.01 vs. pre-test; * P < 0.05 vs. pre-test (paired <i>t</i>-test)]. FMD%, Adropin, and NOx levels, before and after the intervention in both groups. Data are expressed as means ± SD [** P < 0.01 vs. pre-test; * P < 0.05 vs. pre-test (paired <i>t</i>-test)].](https://services.brieflands.com/cdn/serve/3170b/f6a2432b4f1ab13a99255a394e87719585c62f35/asjsm-113566-i001-F1-preview.webp)