1. Background
Bone is a metabolically active tissue, and its regeneration requires continuous reabsorption and formation processes (1). The imbalance between these two processes results in a decrease in bone density, which is the basis of bone fractures. Two-thirds of these fractures occur in women, especially in the femur and lumbar spine areas (2). According to the diagnostic criteria established by the World Health Organization (WHO), there will be an increase of 23 percent in the number of people who are suffering from osteoporosis in the period of 2010 - 2025 (2, 3).
The main point of decreasing the risk of bone fractures in the elderly is to prevent them from osteoporosis at their early ages (3). As a result, researchers are always looking for the best ways to prevent it (4). Although pharmaceutical options are available, long-term use of drugs is limited because of their side effects; thus, researchers are looking for non-medical strategies to replace pharmaceutical approaches (5, 6).
Regular physical activity is a great way to improve bone mineral content (BMC) and bone mineral density (BMD), and it is one of the best options for preventing the development of osteopenia and osteoporosis at different ages. Nevertheless, there is insufficient evidence on the intensity and the length of exercises required to perform these osteogenic stimulations (5, 7, 8).
There are extensive studies in this context, and they have certainly suggested weight-bearing sports as the stimulator of bone formation in comparison to non-weight-bearing sports such as swimming (9, 10). Although water modulates gravity, there are ambiguities about the effects of swimming on the growth of bone, even in comparison to non-athletes (11, 12). Since osteoporosis, despite the risk of fracture, is usually accompanied by cardiovascular and cerebrovascular diseases (12, 13), which are the most important limiting factors for high-intensity or weight-bearing exercises of patients and older people. Therefore besides its high therapeutic benefits, swimming is considered the best option for them, and it is not ignorable (12).
Moreover, a lot of research has been done to compare the effect of weight-bearing sports on the bone density of two key areas (lumbar and hip); but there is no single result to show a sport that can enhance either or both of these areas (14).
Every sport can have different effects on the bone tissue based on its kinetic and kinematic parameters (3, 5, 7). Professional athletes are engaged in exercise from their early ages to adulthood, so the changes in their bone tissue can be confidently attributed to the type of sport which they practice (5). Thus, the reason for choosing these athletes is to measure the effect of each sport with more strength and confidence. Moreover, the research team tried to choose various weight-bearing sports (high-impact, odd-impact, repetitive low-impact, and non-impact exercise loadings) to have a better distinction between them. Nonetheless, it is possible that high-intensity exercise can also have negative consequences, such as the female athlete triad, which may have medical manifestations of eating disorders, functional hypothalamic amenorrhea, and osteoporosis (13, 15).
So, in summary, there are three unknown questions. First, do the swimmers have a stronger bone structure than non-athletes? Second, which one of the weight-bearing sports is more effective than the others? Finally, which areas of the body's skeletal structure are most affected by each sport?
Regardless of the scientific issues about the types of sport, the subjects of most previous studies were either the amateur and recreational athletes or semi-professional ones (16-18); and few studies have examined the elite athletes in various sports (3, 19, 20). To our knowledge, there is no research that only compares the effects of swimming, volleyball, basketball, and long-distance running together on elite female athletes.
2. Objectives
The aim of this study was to compare the bone status (lumbar and proximal femur) of elite female athletes in different sports. In fact, there were two main goals in the present study: First, the comparison of swimming athletes with non-athletes, and second, the comparison of different sports (long-distance running, volleyball, and basketball) with each other.
3. Methods
This cross-sectional study compared the effect of different sports on BMC and BMD of athletes. The Ethics Committee on Sport Science Research Institute, Tehran, Iran, obtained its ethical approval.
3.1. Participants
This study consisted of 58 subjects who voluntarily enrolled in the study; 48 were elite female athletes present in national team camps, and all of the participants gave their informed consent before the study. They were purposefully divided into four groups of long-distance running, volleyball, basketball, and swimming (n = 12 for each) and a control group of 10 non-athletes aged 19 - 25. Initially, participants' personal and demographic information forms had been completed. The control group participated in no organized and regular sports activity during this time and continued the activities of their daily life (ADL) (3). They were selected in almost the same age, height, and weight ranges as the athletes. The athletes were females over 19 years old with more than eight years of experience in their field and at least 12 - 16 hours of training per week. They must compete in high-level championships, which are national or international levels, for a minimum of 4 years (3, 5).
3.2. Exclusion Criteria
All subjects of the study had no female athlete triad (15, 21), hypo or hyperthyroidism, parathyroid, adrenal, diabetes, respiratory heart disease, and kidney or liver failure (13). They neither smoked nor drank alcohol (22). Meantime the athletes suffering from musculoskeletal injuries during the study, for example, six months quitting the sport for fracture or dislocation injury, were excluded from the research (4, 5).
3.3. Procedure Measuring the Mineral Content and Density of Bone
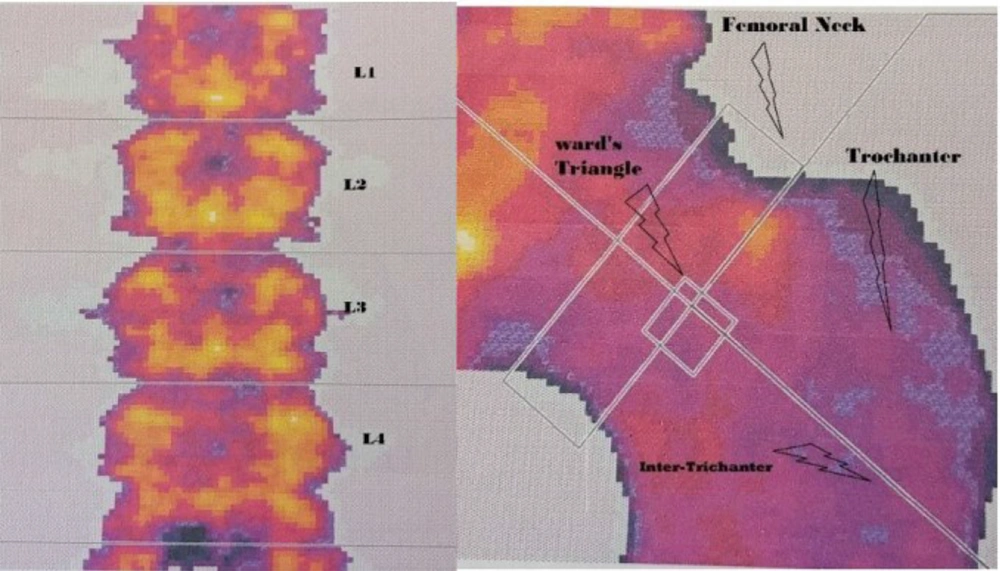
For measuring the BMC (g) and BMD (g/cm2) in the areas of the lumbar spine, (L2 - L4), and proximal femur (neck, trochanter, and Ward’s triangle) (see Figure 1), the DEXA method was applied (Hologic Series Discovery QDR, Software Physician's Viewer, and APEX System Software version 3.1.2. Bedford, MA, USA) as shown in Figure 1. This method is the gold standard for measuring bone density (23). The machine was calibrated every day on the basis of its primary standards, and it was analyzed by the evaluator. The subjects of the study had to wear light clothes, be barefoot, and in supine position on the machine's bed for almost 20 minutes. The error probability of this measurement for BMC and BMD was < 1%. According to WHO definitions, > -1.0 SD shows normal BMD, ≤ -1.0 SD, > -2.5 SD indicates osteopenia, and ≤ -2.5 SD demonstrates the osteoporosis (24, 25). It should be mentioned that lumbar spine and femoral neck areas were measured based on Z-score.
3.4. Statistical Analysis
The statistical analysis was performed using the SPSS v19.0 software (SPSS Inc., Chicago, IL). One-way ANCOVA was applied to compare BMC, BMD, and Z-score adjusted for BMI, percentage of body fat (BF %), and age in five study groups. Post-hoc test of Bonferroni was also used to compare the means. The significant difference level was set at P ≤ 0.05.
4. Results
Table 1 shows the demographic characteristics of the subjects. The results of one-way ANOVA indicated significant differences among the means of groups; each group was compared by the Bonferroni method for post hoc test (Table 1). The homogeneity of variance was confirmed, and we found that the residuals are normally distributed. So, a one-way ANCOVA was conducted to compare the amount of BMC, BMD, and Z-score in five study groups while the values of BMI, BF%, and age were controlled (Table 2).
| Groups | N | Age (y) | Weight (kg) | Height (cm) | BMI (kg/m2) | Body Fat (%) |
|---|---|---|---|---|---|---|
| Basketball | 12 | 20.29 ± 0.38 c, d, f | 61.83 ± 3.46 e, f | 177.63 ± 6.51 c, d, e, f | 19.61 ± 0.84 c, d | 22.80 ± 1.01 c, d |
| Volleyball | 12 | 22.58 ± 1.31 d, e | 62.42 ± 1.38 e, f | 170.58 ± 3.96 | 21.49 ± 1.24 e, f | 25.58 ± 1.64 d, e, f |
| Runners | 12 | 24.21 ± 0.89 e | 64.54 ± 2.76 e, f | 169.67 ± 2.87 | 22.45 ± 1.46 e, f | 27.11 ± 1.84 e, f |
| Swimmers | 12 | 19.92 ± 0.90 f | 55.75 ± 0.97 | 166.75 ± 0.75 | 20.05 ± 0.36 | 23.24 ± 0.61 |
| Controls | 10 | 23.10 ± 1.20 | 55.50 ± 2.68 | 167.55 ± 2.79 | 19.76 ± 0.40 | 23.62 ± 0.39 |
Abbreviation: BMI, body mass index.
a Values are expressed as mean ± SD.
b P ≤ 0.05 significant differences with basketball players.
c P ≤ 0.05 significant differences with volleyball players.
d P ≤ 0.05 significant differences with runners.
e P ≤ 0.05 significant differences with swimmers.
f P ≤ 0.05 significant differences with controls.
Based on the results of the Bonferroni post hoc test, which is shown in Table 2, lumbar spine BMC (L2 - L4), in the long-distance running group is significantly higher than in basketball, volleyball, swimming, and control groups, respectively (P < 0.01). Femoral neck BMC in the basketball group is significantly higher than in long-distance running, volleyball, and control groups, respectively (P < 0.01); this variable in swimming is significantly higher than in volleyball and control groups (P < 0.01). Trochanteric and Ward's triangle BMC of basketball groups is significantly higher than other groups (P < 0.001).
| Variables | Basketball | Volleyball | Runners | Swimmers | Controls |
|---|---|---|---|---|---|
| BMC L2-L4 | 49.70 ± 1.26 d, e | 54.02 ± 1.04 d, e, f | 59.76 ± 1.51 e, f | 45.80 ± 1.37 | 47.71 ± 1.30 |
| BMC femur neck | 6.17 ± 0.20 c, d, f | 4.77 ± 0.16 e | 4.82 ± 0.24 | 5.83 ± 0.22 f | 4.07 ± 0.21 |
| BMC trochanter | 15.94 ± 0.38 c, d, e, f | 9.26 ± 0.32 f | 8.24 ± 0.46 | 8.03 ± 0.42 | 7.26 ± 0.39 |
| BMC Ward’s triangle | 3.22 ± 0.04 c, d, e, f | 0.71 ± 0.03 d, e | 0.90 ± 0.04 | 0.91 ± 0.04 | 0.75 ± 0.04 |
| BMD L2-L4 | 1.19 ± 0.01 e, f | 1.16 ± 0.02 d, e, f | 1.26 ± 0.02 e, f | 1.09 ± 0.02 | 1.04 ± 0.02 |
| BMD femur neck | 1.29 ± 0.02 c, d, e, f | 0.96 ± 0.02 d, f | 1.10 ± 0.02 e, f | 0.94 ± 0.01 | 0.85 ± 0.02 |
| BMD trochanter | 1.10 ± 0.02 c, d, e, f | 0.77 ± 0.02 d | 0.92 ± 0.02 e, f | 0.71 ± 0.02 | 0.71 ± 0.02 |
| BMD Ward’s triangle | 1.17 ± 0.03 c, d, e, f | 0.86 ± 0.03 e, f | 0.89 ± 0.03 e, f | 0.70 ± 0.02 | 0.78 ± 0.03 |
Abbreviations: BMC, bone mineral content (g); BMD, bone mineral density (g/cm2).
a Values are expressed mean ± SD.
b P ≤ 0.05 Significant Differences with basketball players.
c P ≤ 0.05 Significant Differences with volleyball players.
d P ≤ 0.05 Significant Differences with runners.
e P ≤ 0.05 Significant Differences with swimmers.
f P ≤ 0.05 Significant Differences with controls.
Lumbar spine (L2 - L4) BMD of long-distance running, basketball, and volleyball groups is significantly higher than in swimming and control groups, respectively (P < 0.05), and in long-distance running, it is significantly higher than volleyball group (P < 0.001). Femoral neck BMD of basketball and long-distance running groups are significantly higher than in volleyball, swimming, and control groups, respectively, while this is significantly higher in basketball than long-distance running (P < 0.01). Trochanteric BMD of basketball and long-distance running groups are significantly higher than in volleyball, swimming, and control groups (P < 0.01). Ward's triangle BMD of the basketball group is also significantly higher than long-distance running and volleyball, control, and swimming groups (P < 0.001).
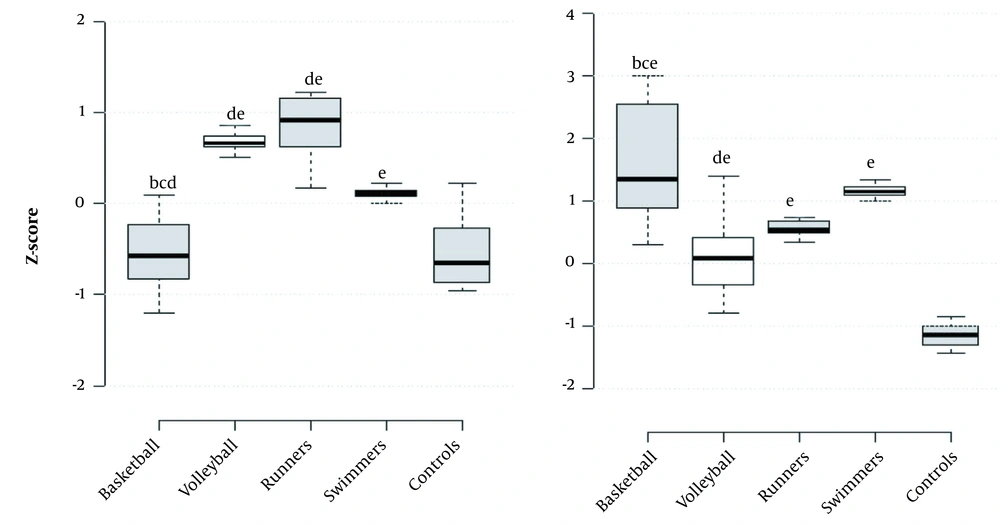
Based on the results shown in Figure 2, lumbar spine (L2 - L4) Z-score of basketball and control groups is significantly lower than long-distance running, volleyball, and swimming groups, respectively, and volleyball players and long-distance running have significantly higher values than swimming (P < 0.001). Femoral neck Z-score of basketball group is significantly higher than long-distance running, volleyball, and controls respectively, it is significantly higher in swimming, long-distance running and volleyball groups than controls respectively, and in swimming, it is significantly higher than volleyball players (P < 0.001).
Box plots of (left) L2- L4 Z- score and (right) femur neck Z- score (a, P ≤ 0.05 significant differences with basketball players; b, P ≤ 0.05 significant differences with volleyball players; c, P ≤ 0.05 significant differences with runners; d, P ≤ 0.05 significant differences with swimmers; e, P ≤ 0.05 significant differences with controls).
5. Discussion
The professional sport improves BMC and BMD (3, 26, 27). In general, the present study reconfirms the effect of weight-bearing sports (basketball, long-distance running, and volleyball, respectively) on bone development (3, 26-28). It also confirmed that non-weight bearing sport (swimming) at professional levels improves bone health compared to the healthy non-athlete group (11).
There are some differences in the present study, and it adds several novel aspects to the literature of this context. The focus was first on the BMD and the BMC of the proximal femoral regions of these athletes. The results showed that the basketball players have higher BMC and BMD values in these areas in comparison to the athletes of other sports, and swimmers have better bone status than non-athletes. Among these athletes, the runners were relatively superior to the volleyball players. So far, in this study, it is certainly considered that basketball is more effective than other sports in this area.
Then the BMD and BMC of these athletes in the lumbar spine area were examined; and its results showed that the long-distance runners gained more significant BMD and BMC values than other athletes in this area, while the basketball players had a denser lumbar spine than the volleyball players. In this area, the same as the femoral area, the swimmers had relatively higher bone density than non-athletes. Hence, in this study, an endurance runner is definitely identified to have a stronger lumbar spine compared to other athletes.
According to the definition of the WHO, there is another valuable aspect of assessing the bone status of individuals: Their comparison with the reference population of the same sex and age, which is called the Z-score (24). For this reason, this criterion was evaluated in the present study to rely more confidently on the obtained results. The obtained results of athletes in two high-risk fracture areas (femoral neck and lumbar spine) (2) were interesting.
The values of Z-score for the femoral neck area of all athletes are set approximately between 0 and 1.5, while it is less than -1 for the control group. It indicates that the entire athletic population has higher bone density than average, and the control group is more prone to osteopenia. The results also reveal that the swimmers not only have higher numbers than non-athletes in this index, but their values are also more than volleyball players, and even almost equal to the runners, but still the basketball players had significantly higher scores than the other athletes in the femoral neck area.
The more interesting result in this context is the bone density of swimmers in the lumbar spine area, which is higher than the non-athletes and even the basketball players. The bone density of this area in the runners and the volleyball players is significantly higher than all groups, such that their Z score is close to 1, and the runners are slightly superior to the volleyball players.
In order to answer the first main goal of the study, the results of the Z score definitely proved that swimming is effective for bone density improvement compared to non-athletes. Recently, a systematic review provided limited evidence on the benefits of swimming to improve BMD values, which is inconsistent with the results of the present study (12), while another systematic review was completely consistent with this study and found the mentioned sport useful for improving the bone health in comparison to non-athletes (11). Nonetheless, most of the research that is done in this context is contrary to the present reports, and the neutral or ineffective results of swimming on BMC and BMD had been suggested by them (10, 17, 18, 21, 29-32). It must be mentioned that the under-study subjects of all the above disparate researches were non-professional athletes, which can justify the differences between the present studies' findings with theirs.
Moreover, in order to obtain the conclusion for the second main goal, several aspects are explained separately. The first case is related to the weight-bearing sports; if the effect of each sport on improving the skeletal position of the lumbar spine is the priority of study, the running has the first priority, and then volleyball and basketball are chosen, respectively. In the second part, if it is focused on choosing the sport that increases the bone health of proximal femoral regions, basketball will be the first priority, and running, and volleyball will follow, respectively. Finally, in a general view, basketball can be considered the superior, long-distance running as the second, and volleyball as the third osteogenic sport in both body parts.
There are some differences in how these sports are performed, which explains the main reasons for bone changes in these athletes. For example, in long-distance running, due to the proximity of the lumbar spine to the body's center of gravity, the body's posture is tolerated better for a long time. So the repetitive applied loads of the earth’s reactionary forces can be the possible reason for the lumbar spine strength in these athletes (28).
In contrast, the dominant motion patterns of basketball players include short and explosive runs with sudden changes of direction and jumps. This condition can apply a constant and increased load on these players' hip and femur areas (33, 34), which probably is a reason for affirming the effect of this sport on the development of bone tissue in mentioned areas.
On the other hand, the dominant movement in volleyball is jumping and changing sudden directions without clashing with the opponent and repetitive load (27, 33). As a result, it is true that these athletes have less BMD and BMC in both lumbar and femoral areas than runners and basketball players, but there is a greater balance in bone position between these two areas.
However, recently, some researchers have definitely considered endurance running as an effective exercise in the process of bone marrow cell uptake, which is fully consistent with the findings of the present study. These researchers stated that the lack of adequate rest and proper recovery in these athletes is the reason for their bone tissue damage (28). In contrast, some other researchers indicated that endurance runners have the lowest BMD values in every part of the body (total-body, lumbar spine, and pelvis) in comparison to gymnasts, softball players, and even swimmers, except in the average leg score, which is a sign of incompatibility with the present study (35).
In 2020, a systematic review compared the BMD values in the athletes of basketball, volleyball, football, and swimming and a non-athlete group, where basketball was considered the best osteogenic sport in all areas of the body, especially the lower limbs, that is compatible with the results of this study (27). In another study, investigators reported that the sports of volleyball and basketball, respectively, are effective in improving the bone condition of prepubescent boys, especially in the femoral neck area. This result is inconsistent with the present study in terms of the superiority of volleyball players (33). However, the subjects of this study were not in the same age and level of activity; hence, these differences necessitate the importance of more detailed studies in the future.
The strengths of the present study can be mentioned in two parts: (1) the first is related to the participants of the study, which included female athletes with high performance on the national teams who were trained to participate in international competitions; (2) the second is the selection of four sports in different exercise loadings (high-impact to non-impact) for the accurate identification of the effects of each sport on the bone tissue of its athletes.
The limitations of the present study that could affect the results are as follows: (1) the first limitation is the small number of subjects, which happened due to the limited access of the research team to elite athletes; (2) the second is related to the type of study, which was a cross-sectional study, and the team members were not able to follow up on its results; (3) the third was the impossibility of checking the nutritional status of subjects for the researchers (25).
And the last was the lack of control over the training programs of the athletes; for example, the level of weight-bearing and resistance exercise that the swimmers perform in their daily training program was not clear for the research team. Therefore, it is highly suggested that the researchers consider these limitations as much as possible for future studies and that they study the elite athletes of other popular sports such as football besides the sports of the present study.
5.1. Conclusions
In summary, the basketball players (in proximal femur areas) and the long-distance runners (in lumbar) had the highest BMD and BMC values among other groups; while the volleyball players (in the proximal femur and lumbar areas) had higher BMD and BMC values than swimmers and non-athletes; and finally, the swimmers were superior to the non-athletes in both areas. So the, basketball and long-distance running are considered the best osteogenic sports in the prevention of osteoporosis.