1. Background
Metabolites and metabolic adaptations have an important effect on the performance of athletes, and research on these factors has always been of interest to researchers (1). Carbohydrate and lipid-rich diets are recommended for endurance exercise. Carbohydrates, as a factor in the delayed onset of fatigue, are associated with increased muscle glycogen stores. Also, lipids that originate from the fatty acids of adipose tissue, intramuscular triacylglycerides (IMTG), and cholesterol are used as a fuel source for energy stock during the time of submaximal exercise (2, 3). Many hormones and enzymes are involved in the metabolism of carbohydrates and lipids. Betatrophin or angiopoietin-like protein (ANGPTL8) plays a double role in the regulation of triglyceride (TG) and glucose metabolism (4, 5). The ANGPTL8 gene is expressed in the liver; next, the betatrophin protein is released in the blood (6). Betatrophin has been proposed as a possible mediator of the increased β-cell duplication in diabetic patients (7, 8). Despite the recent publications that have manifested, betatrophin was increased in patients with type 2 diabetes (T2D) (9, 10), others reported that betatrophin was decreased in patients with T2D (11). However, the levels of betatrophin decrease during starvation and lead to an increase in lipoprotein lipase (LPL) levels to provide the required energy for the muscles and the entire body through lipid metabolism (12). In addition, there is a reverse correlation between IMTG content and insulin sensitivity in the obese and people with T2D, but a paradox in endurance athletes is that although they have very high insulin sensitivity, their IMTG content is also high (13, 14). Besides, betatrophin is a stress-response protein that regulates the metabolism of lipids by suppressing the expression of adipose triglyceride lipase (ATGL), which indicates a regulation mechanism between betatrophin and lipid hemostasis in mammalian cells (15).
In general, betatrophin is involved in carbohydrates and lipid metabolism under various nutritional conditions. Likewise, any substance that can affect the levels of betatrophin in different nutritional conditions will be able to change the metabolism of carbohydrates and lipids. In this study, for the first time, we investigated the effect of propolis as a herbal supplement along with exercise on betatrophin alterations.
Propolis has shown great therapeutic effects and is widely used in the food and medicine industry. Its biological effects include anti-inflammatory (16), antibacterial (17, 18) antifungal (19), antioxidant (20), antidiabetic (21), and anticancer activities (22); it is also known as an immune system booster (23). In addition, propolis has a strong antioxidant activity. It contributes to lipid metabolism so the use of herbal and natural supplements improves the side effects of reactive oxygen species (ROS) production in athletes (24).
The serum betatrophin changes have only been studied under clinical conditions such as diabetes and fatty liver, and there is no clear information on the changes of betatrophin in athletes. The previous studies have reported controversial findings and showed different expression patterns of betatrophin in individuals with obesity and T2D, but the consideration of propolis’ role in improving diabetes suggests increased betatrophin by propolis supplementation to be associated with improved carbohydrate and lipid metabolism (21, 24, 25).
2. Objectives
Therefore, to address this issue, we evaluated the weight of participants after the changes in the betatrophin serum levels to achieve a better understanding of the role of betatrophin in carbohydrate and lipid metabolism.
3. Methods
This study was a semi-experimental and applied research, including pretest and posttest phases.
3.1. Participants
Forty-eight male professional athletes from long-distance running and physical fitness teams of Qazvin city were selected. All participants were verbally informed about the study, and those that agreed to their data being used signed a written informed consent form. This study was approved by the Ethics Committee of Qazvin University of Medical Sciences (Ethical code: ir.qums.rec.1397.407).
Of the 48 participants, 44 met the inclusion criteria and consented to have their data included. Eligibility criteria for the participants were identified as healthy, non-smokers, and had no history of pulmonary, heart, metabolic diseases, lipid profile disorders, and hypertension. At the beginning of the study, athletes were told that they should not use any supplements for one month before the start of the study; also, they would be excluded from the study if they took extra supplements.
The study participants have included a mean age of 22 ± 3 years, mean height of 177.5 ± 6.5 cm and mean weight of 76 ± 6 kg. The diet of athletes was monitored by the nutritionist through an oral recall form one month before the start of the study until the end of the study. Athletes' dietary and energy intake data were collected using a 24-hour feed-in questionnaire.
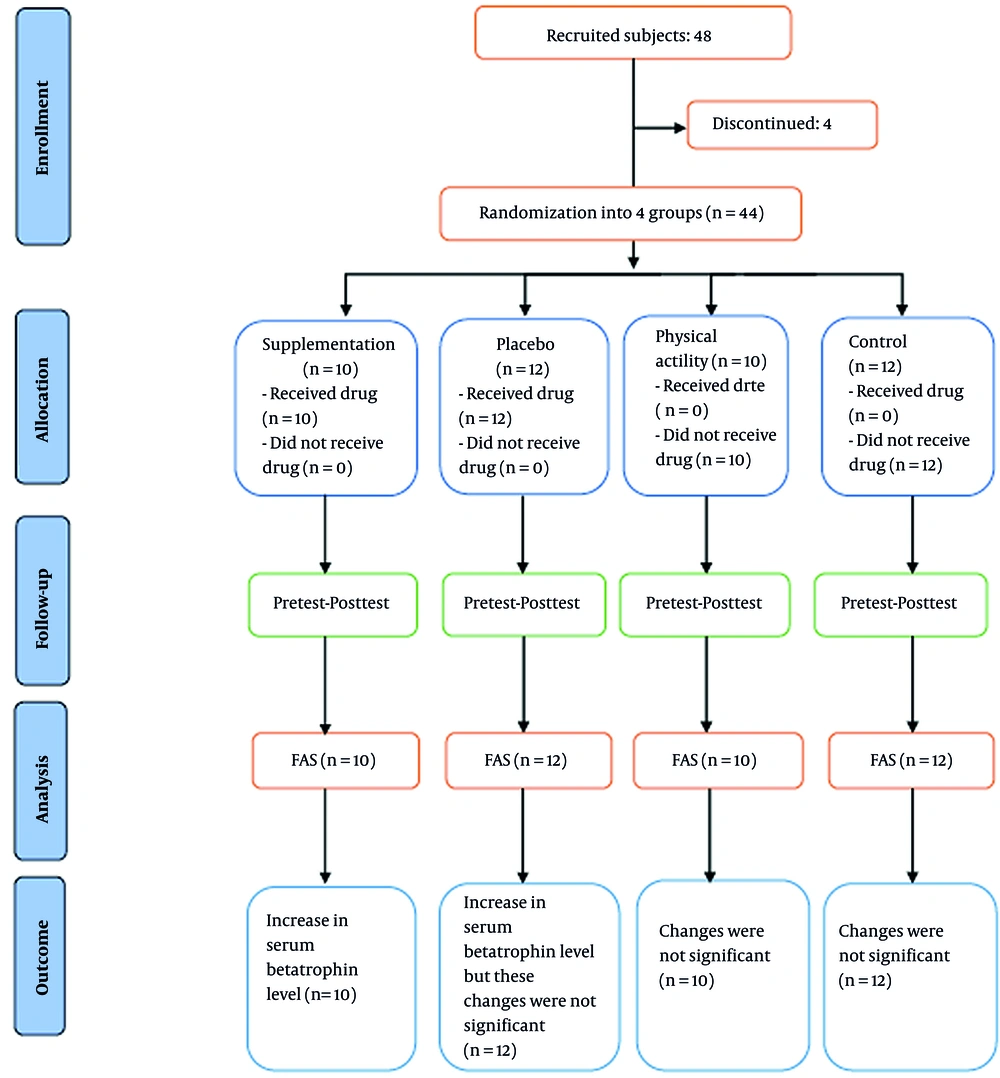
In the following, 44 participants were randomly divided into four groups consisting of supplementation (n = 10), placebo (n = 12), physical activity (n = 10), and control (n = 12), (Figure 1).
3.2. Assessments
One month after the diet, without any supplementation and before the intervention, the serum betatrophin levels of the participants were collected (pretest phase). Next, the supplementation and placebo groups received two 500 mg capsules of propolis supplementation and cellulose, respectively (daily, one after lunch, and one after dinner) according to the Samadi protocol with some modification (26). The capsules given to the placebo group in terms of shape and color were similar to those given to the supplement group; the cellulose capsules had no specific properties, aroma, or taste. The physical activity group did not take any supplementation, and the control group included healthy participants with no history of exercise; also, the serum betatrophin levels of the participants were measured four weeks after the intervention (posttest phase) (Figure 1).
3.3. Testing Procedures
The blood samples (5 mL) were taken from all 44 participants once as a pretest in the morning, after at least 11 hours of fasting in a sitting position, and once as a posttest after four weeks of intervention in a fasting state (blood sampling conditions were the same in pretest and posttest).
The blood samples were separated by centrifugation at 3000 rpm for 15 minutes at -4°C and maintained at -80°C until biochemical analysis. The serum levels of betatrophin were measured using an ELISA kit (MyBioSource, Germany). The inter- and intra-assay coefficient of variation was 8% and 10%, respectively; the sensitivity of the kit was 2.8 ng/dL.
3.4. Statistical Analysis
The Kolmogorov-Smirnov test is used to assess the normality of the data and the results were expressed as mean ± standard deviation. ANCOVA was applied for inferential statistics. The statistical analyses were performed at the significance level of 0.05 using SPSS version 20.
4. Results
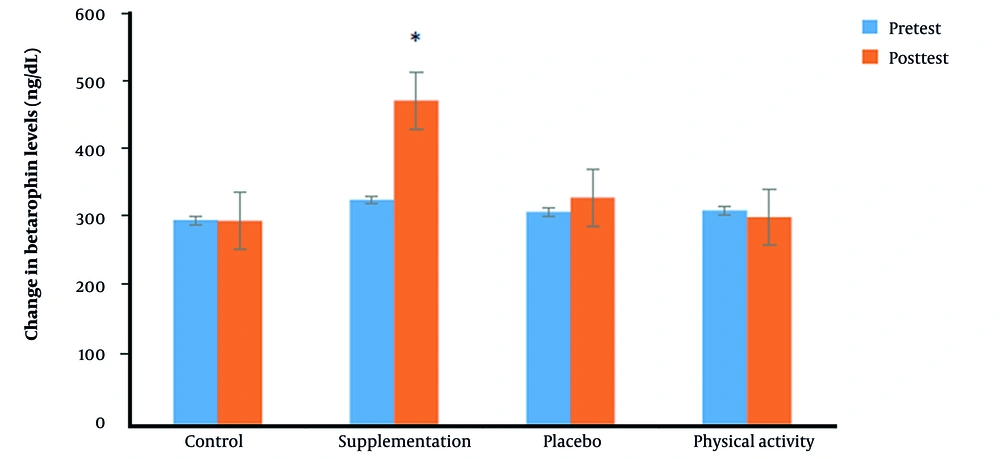
According to the results presented in Table 1, there was no significant decrease in the serum levels of betatrophin in three groups of the placebo, physical activity, and control in the intragroup comparison between the pretest and posttest (P > 0.05); the difference was significant only in the supplementation group (P = 0.001). In the intergroup comparison, betatrophin serum levels increased significantly in the supplementation group compared to the other three groups (P = 0.001). In the placebo group, serum levels of betatrophin also increased, but the difference was not significant. On the other hand, in the intragroup comparison, the weight difference was significant only in the supplementation group (P = 0.027), but in the intergroup comparison, this difference was insignificant (P = 0.132). Figure 2 shows the alteration in serum levels of betatrophin.
| Variables & Groups | Frequency | Pretest | Posttest | t | P (Within Group) | P (Between Group) |
|---|---|---|---|---|---|---|
| Betatrophin (ng/dL) | 0.001 a | |||||
| Control | 12 | 300.87 ± 50.59 | 299.85 ± 45.44 | 0.057 | 0.956 | |
| Supplementation | 10 | 330.70 ± 35.39 | 476.19 ± 59.7 | 9.428 | 0.001 a | |
| Placebo | 12 | 313.37 ± 45.93 | 334.05 ± 14.29 | 1.680 | 0.121 | |
| Physical activity | 10 | 315.20 ± 22.14 | 305.22 ± 15.71 | 0.485 | 0.639 | |
| Weight (kg) | 0.132 | |||||
| Control | 12 | 74.33 ± 1.23 | 74.25 ± 1.13 | 1.000 | 0.339 | |
| Supplementation | 10 | 73.75 ± 7.7 | 73.45 ± 7.50 | 2.548 | 0.027 a | |
| Placebo | 12 | 78.60 ± 4.88 | 78.3 ± 4.97 | 1.964 | 0.081 | |
| Physical activity | 10 | 77.80 ± 6.87 | 77.80 ± 6.87 | 1.000 | 0.343 |
Comparison of Changes in Measured Variables Before and After Four Weeks of Intervention
5. Discussion
Propolis is a natural material that is formed from the secretion of the wax and parotid glands of bees using substances gathered from the buds and bark of plants (27) Propolis has great therapeutic effects and has been widely used in the food and drug industries (27). Betatrophin is a newly uncovered adipokine that plays a major role in energy homeostasis, and carbohydrate and lipid metabolism (4).
In this study, the authors intended to investigate the modifications of betatrophin serum levels by exercise training and the use of propolis that can work as a supplement in athletes. For this purpose, the intake of the supplementation propolis, which has been known to have effects on various physiological activities, and training were applied to 44 male professional athletes for four weeks. Blood sampling was performed twice (pretest and posttest). Next, the serum levels of betatrophin (as a possible target of propolis) were measured by ELISA.
As a result, the serum levels of betatrophin increased after four weeks of long-term endurance training with propolis supplementation, and endurance training alone had no significant effect on the serum levels of betatrophin in the placebo and physical activity groups.
It considers that this can result from the enhancement effect of propolis on serum levels of betatrophin, and consequently, the metabolism was improved, and a decrease in weight was induced. A study on serum betatrophin levels after four weeks of endurance training in diabetic rats showed that endurance exercise alone did not cause significant changes in serum betatrophin levels. However, it had positive effects on pancreatic tissue in diabetic specimens (28).
Although no studies have examined the effect of propolis on betatrophin levels, several studies have investigated the influence of propolis in the treatment of diabetes. In this way, a study has shown that propolis consumption (daily 900 mg) for 12 weeks could improve blood glucose and some lipid parameters in patients with T2D; however, no significant effects were observed on insulin resistance parameters (26). In research by Tang et al., the antidiabetic effects of propolis led to a significant decrease in insulin resistance in T2D rats with high-fat diet-fed (21); similar results were also reported by Kitamura et al. and Fukuda et al. (25, 29). Therefore, concerning the relationship between betatrophin and propolis with glucose metabolism, propolis has increased the serum level of betatrophin in the participants of this study.
On the other hand, betatrophin is involved as a new potent improver of glucose tolerance in patients with insulin resistance (30). Although recent reports investigating a betatrophin link to glucose metabolism showed contradictory data, which seems is mainly due to differences in the sample size and the examination of diverse types of betatrophin by different kits (31, 32). For example, Abu‐Farha et al. showed that serum betatrophin levels were significantly reduced in T2D patients compared to non-diabetics (32). A further study by Gomez-Ambrosi et al. reported that concentrations of circulating betatrophin diminished in obesity and T2D subjects (33). These studies are consistent with our theory of the role of betatrophin in increasing the carbohydrate and lipid metabolism of athletes. However, another study showed that circulating levels of betatrophin were augmented in subjects with T2D compared to subjects without T2D (10), which this increase may be associated with increased insulin resistance and demand for insulin in T2D subjects. In consistence with our findings, Quagliarini et al. have also shown that betatrophin is involved in the metabolism of TG and fatty acids by ANGPTL3 (34). Furthermore, recent research has shown that increased betatrophin by exercise and physical activity leads to an increase in brown fat and weight loss (28).
The barriers to the current study included the lack of references to compare the results; all the previous studies were only focused on obese and diabetic participants, and no research was conducted on healthy athletes to compare, and we applied their results. So, it is possible, that the cycle of changes in the betatrophin serum levels in healthy and athletic participants is different from that of individuals with obesity and diabetes. Therefore, further studies are required in this regard.
5.1. Conclusions
In conclusion, we have shown the first evidence that endurance training plus propolis supplementation increases the betatrophin serum levels of male athletes. Our findings also suggest a potential association between the betatrophin serum levels and loss of weight that indicates betatrophin involvement in carbohydrate and lipid metabolism. In addition, the propolis-mediated changes in betatrophin levels can be a fundamental approach in obesity-related disorders, including blood pressure. However, due to the lack of similar research for the comparison of the results, additional studies are needed to elucidate the role of betatrophin in athletes' health.