1. Background
The discovery of myokines has emphasized the important role of muscle as an endocrine organ that communicates with other tissues (e.g. fat tissue, pancreas, and liver) to regulate metabolism through different pathways. A recently discovered muscle-derived factor called irisin, a small protein cleaved and secreted from fibronectin type III domain-containing protein 5 (FNDC5), was found to activate fat browning and thus, inducing thermogenesis and improving body weight and glucose homeostasis (1). As a consequence of this promising result irisin became a therapeutic target for the treatment of obesity and metabolic diseases in humans (1, 2).
The existing research literature shows contradictory results on serum irisin levels in response to exercise in humans. One stream of research suggests that exercise causes transient irisin increase when interventions are acute (one-bout) and intensity is moderate-high (3-5). Conversely, recent studies reported either significant decreases (6) or no changes (7) when high-intensity resistance training was performed. A second stream of research suggests that results might differ when studying the effects of longer exercise intervention periods (i.e. eight weeks) in baseline irisine levels. In this regard, some exercise intervention studies found no changes (8-12), while others found variable effects of exercise in irisin concentrations depending on mode (e.g. aerobic vs. resistance training) and intensity (e.g. moderate vs. high) (1, 13, 14). It seems that factors such as age, (7, 12) diet, body composition (e.g. body mass index (BMI), and lean body mass (LBM)) (12, 15, 16), physical activity levels (11, 16, 17), health status (18, 19), or day-night rhythm variation (20) may influence either irisin baseline levels or its response to exercise. For example, irisin has been found to have a 74% day-night variation without exercise exposure (20). Therefore, controlling for age, body composition, and diet, and including matched controls assessed as the intervention group, is a recommended strategy to address these confounding variables (21). Interestingly, only a few studies examining the effects of exercise in healthy adults include a control group, with one of them reporting significant changes in irisin levels in the control group (4, 12, 14, 17, 22).
2. Objectives
Given the results reported in the literature, the aim of this project was two-fold: First, we aimed to analyze the acute response of irisin to one-bout of high intensity resistance training, and the responses of baseline levels of irisin, body composition, and strength to a three-week high-intensity resistance training program, compared with age, BMI, and LBM matched non-exercise controls. Second, we aimed to analyze the association between baseline irisin levels and physical fitness, physical activity, and dietary habits.
3. Methods
3.1. Sample and Design
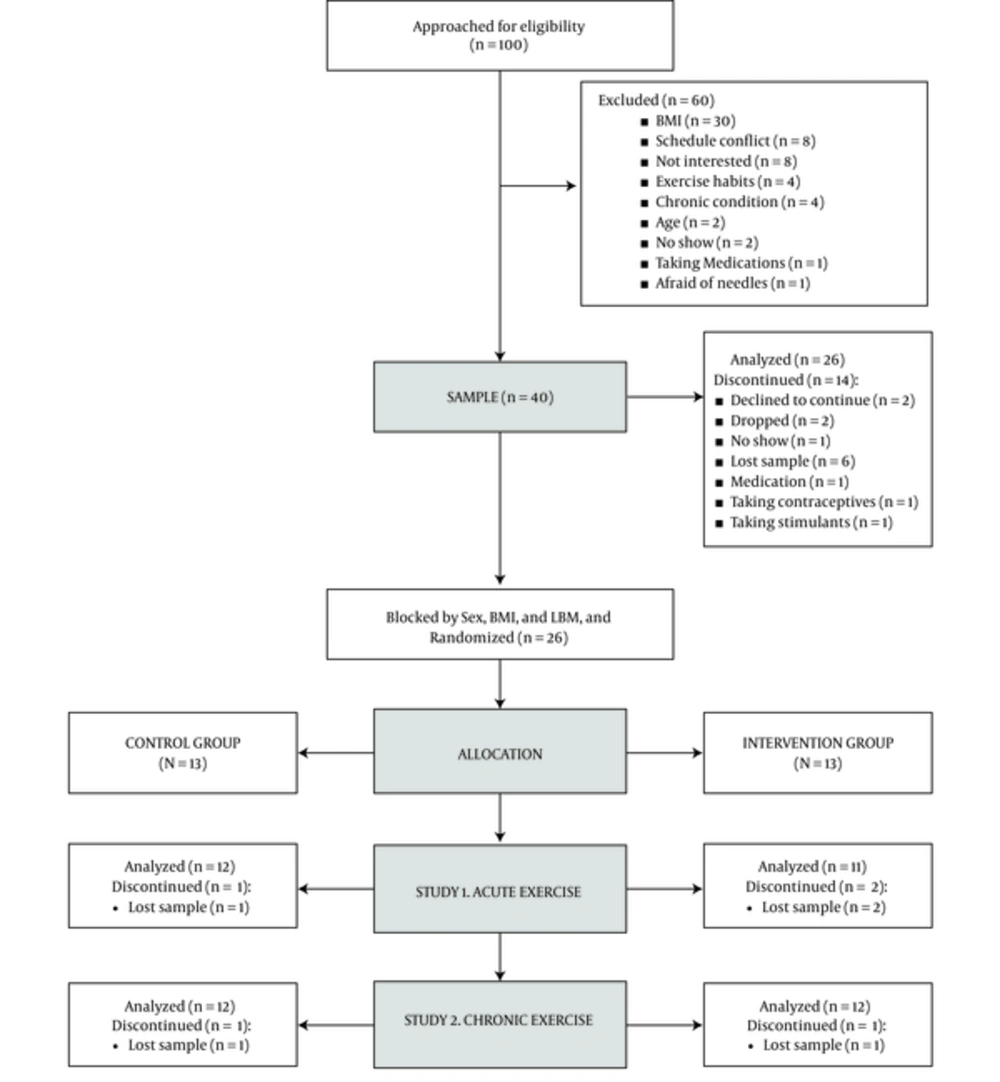
The study was approved by the Institutional Review Board (approved protocol ID: #504792) and was carried out in accordance with the declaration of Helsinki. A written consent form was obtained from each participant prior to the start of the study. The intervention was carried out between January 2015 and July 2015. Participants were included in this study following the CONSORT guidelines to randomized controlled trials (23). This study was registered at www.clinicaltrials.gov (NCT02478658 and NCT02475473).
The sample size was calculated to detect changes in serum irisin concentration according to the expected magnitude of the effect (d = 0.725) that was previously reported in the literature by Daskalopoulou et al. (2014) Assuming a power of 80%, and a significance level of 0.05, 17 participants would be necessary to detect a 2-tailed difference between matched pairs. Preparing also for a 20% dropout, it was determined that 42 subjects (21 in each arm of the study) should be enrolled (Figure 1).
A total of 100 Caucasian males and females were contacted for pre-screening to participate in this study, as shown in Figure 1. Inclusion criteria were as follows: 18-30-years old with a normal BMI (18.5 - 24.99 kg/m2). Exclusion criteria: a, cardiovascular, metabolic, or pulmonary diseases or conditions; b, being pregnant; c, taking medications that affect endocrine or cardiovascular function; d, hypertension; e, being engaged in low intensity strength training more than two times per week or moderate-high intensity training of any type and frequency; f, cigarette smoking. Discontinuance criteria: a, failure to maintain adequate compliance of the study sessions or cooperation with the researchers during the visits, and; b, no longer meeting the criteria.
After pre-screening, 40 participants were enrolled. From these, 5 participants declined to continue for personal reasons, 3 began on incompatible medications, and 6 serum samples were not obtained for technical reasons (6 samples were not obtained due to complications during venous access). The final sample comprised a total of 26 participants (n = 14 males, and n = 12 females). Participants were randomly allocated into intervention and control groups equally distributed by sex. Block randomization was used (block size = 4) to homogenize by BMI and LBM. Stata statistical software was used to program the randomization algorithm. A total of 23 participants (control, n = 12) were analyzed in the one-bout phase, and 24 participants (control, n = 12) were analyzed for the 3-week phase (Figure 1). Furthermore, 5 participants (intervention group) from the 3-week phase were included for an additional sub-analysis.
3.2. Assessments
3.2.1. Physical Activity and Dietary Assessments
Participants were required to maintain their normal physical activity while wearing an accelerometer-based activity monitor for seven days. Actical (Respironics Inc., PA, USA) methodology details and validation are provided elsewhere (24). Dietary habits were assessed using the Automated Self-administered 24-hour recall (ASA24h) at baseline to ensure no differences in dietary habits in the sample (25).
3.2.2. Familiarization, Strength and Cardiorespiratory Assessments
During the pre-assessment week, and prior to beginning the intervention, all subjects underwent a familiarization week in order to minimize the learning effect, ensure the use of the correct technique for each exercise, prevent injuries, and train the participants on the use of the OMNI-resistance exercise scale 0 - 10 (OMNI-RES) for perceived exertion (26). Each of the three familiarization sessions consisted of a warm up and a cool-down, and a conditioning phase consisting of 2 - 3 sets of 5 - 8 repetitions of non-weighted exercises used during the intervention period. To determine the absolute maximal muscular strength, a 1 repetition maximum (1RM) test was performed on leg press (1RMLP) and bench press (1RMBP) (27). Leg press-to-body weight (LP-to-BW) and bench press-to-body weight (BP-to-BW) ratios were calculated to obtain strength relative to body weight (28). Cardiopulmonary fitness was determined at baseline via maximal oxygen consumption (VO2max) measured breath by breath via open gas circuit (MedGraphics Ultima, CardioO2, St. Paul, MN), following the standard Bruce protocol (29).
3.2.3. Body Composition and Anthropometric Assessments
Dual-energy x-ray absorptiometry (DXA) was performed to obtain fat mass content (FM), relative body fat (%BF), and LBM content in accordance with manufacturer recommendations (GE Lunar Prodigy, Madison, WI, USA). In addition, weight and height were obtained from all participants. BMI was calculated as weight/height2 (kg/m2). Both DXA and anthropometry were assessed at baseline for the one-bout intervention study, and at baseline and after the 3-week intervention study.
3.2.4. Blood collection and Analysis
Blood samples were drawn from an antecubital vein 15 minutes prior to the intervention session, during the session (45 minutes or at the end of the second circuit), and immediately post-session (before the cool down phase) at session 1 for the acute study. Blood was collected prior to the session at sessions 1, 3, 6, and 9 for the 3-week intervention study. In addition, blood samples were collected in a sub-set of participants (n = 5) to assess intra-session changes during the 3-week intervention - prior, during, and immediately post-session (before the cool down phase) - at sessions 1, 3, 6, and 9 in the intervention group. Participants were requested to ingest a standard diet the days of blood collection. Blood was processed inmediately after collection following the recommendations. The supernatant was transferred to clean 1.5 mL tubes, and serum was stored at -80°C until analysis. All serum samples were treated following the same procedures across the study (30). Serum irisin was quantified using a commercially available ELISA kit (Cat. no. AG-45A-0046YPP-KI01, Adipogen Life Sciences, Liestal, Switzerland). The sensitivity of the assay was 1 ng/mL and the assay range 1 - 50 ng/mL. Intra- and inter-assay variations were < 8.2% and < 9.8%, respectively. The samples were diluted 1/4 with assay buffer, and all samples from a particular subject were analyzed using the same plate (intra-assay). In addition, glucose, lactate (La), and hematocrit (Htc) were determined via finger prick using a Accu-Chek Active glucometer (Roche, Switzerland), a lactate reader (Lactate Plus, Nova Biomedical, Waltham, MA, USA), and a micro-hematocrit IEC MB Centrifuge and micro-capillary reader 275 (Damon / IEC Division, Needham, MA) respectively. Glucose was measured once at baseline. Assessments of La and Htc were performed at session 1 for the acute-intervention study and at sessions 1, 3, 6, and 9 for the 3-week intervention study.
3.3. Interventions
3.3.1. One-Bout and Three-Week Interventions
Participants allocated to the intervention group participated in a supervised circuit training session (one-bout) and/or 3 weeks of circuit training three times per week. The instructor-to-participant ratio was 2:1 for every training session. The sessions were performed between 3:00 - 7:00 p.m., and lasted 50 - 60 minutes approximately. Warm-up consisted of dynamic exercises involving all major muscle groups, and conditioning session included 7 strength exercises engaging the major muscle groups (leg press (LP), rows, back squats, weighted crunches, deadlifts, bench press (BP), and squat jumps with weights) followed by stretch exercises. The participants performed 3 sets of 10 repetitions with resting periods of 30 seconds between exercises, and 2 minutes between sets. The overall OMNI-RES per set ranged 8 - 10, ensuring a minimum intensity of 70% - 80% of 1RM per set (26). The load was changed depending on the participants’ perception when needed. In addition, the intensity was monitored assessing La concentrations at one-bout intervention, and at the end of sessions 1, 3, 6, and 9 for the three-week intervention study.
3.3.2. Control Group
Participants allocated in the control group did not participate in the circuit training program. However, they were required to attend the same number of sessions and stay in a quiet room in the same conditions of temperature, and humidity, and during the same schedule (3:00 - 7:00 p.m.) as the intervention group.
3.4. Statistical Analysis
All data were checked for normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests. In order to detect potential confounding variables, equivalence of control and treatment groups at enrollment was analyzed. Sample characteristics such as age, height, BW, BMI, %BF, LBM, FM, daily calorie intake, and physical activity were compared through two-tailed Student’s t-tests for independent samples. Association of baseline irisin levels with prior physical fitness indicators, physical activity levels, and diet were assessed using Pearson product-moment correlation coefficients.
For the one-bout intervention study, a 2 × 3 (group × time) repeated measures analysis of variance (ANOVA) was used to assess the acute effects of single-bout high intensity resistance training (control vs. intervention) on serum irisin concentration along three repeated measures at pre, 45-minutes, and post on irisin levels. The effects of the acute session on La levels was assessed using a 2 (group) × 2 (pre vs. post) repeated measures ANOVA.
For the 3-week intervention study, a 2 × 4 (group × time) repeated measures ANOVA was used to assess the progressive effects of 3 weeks of high intensity resistance training (control vs. intervention) on irisin pre-session levels along four repeated measures (sessions 1, 3, 6, and 9). A 2 × 5 (group × time) repeated measures ANOVA was used to assess changes in La levels during 3 weeks of training from baseline (pre-session 1) to post-exercise (post-sessions 1, 3, 6, and 9). A 2 × 2 (group × time) repeated measures ANOVA model was used to assess the effects on muscle strength and body composition (pre-assessment vs. post-assessment). Bonferroni-corrected pairwise comparisons were performed as appropriate (31). Additionally, a descriptive sub-analysis was performed to explore the acute response to exercise (pre vs. 45 minutes vs. post) over training sessions 1, 3, 6, and 9. Generalized eta squared (ηG2) and Cohen’s d values were reported to provide an estimate of standardized effect size. The significance level was set to P < 0.05, and all data were expressed as mean ± standard deviation (SD) unless stated otherwise.
Statistical analyses were preformed using Stata 13.1 (StataCorp, College Station, TX) and SPSS 17.0 - statistical package for the social Sciences version 17.0 - for Macinthosh (SPSS Inc., Chicago, IL).
4. Results
A total of 26 participants, 14 males (21.18 ± 1.93 years) and 12 females (21.35 ± 2.52 years), successfully completed the pre-assessment phase. Body composition differences between groups at enrollment in height, BW, BMI, %BF, LBM, and FM are shown in Table 1. No statistically significant differences between groups were found in EI (P = 0.756), protein (0.249), total fat, (P = 0.632), CHO (0.568), added sugars (0.785), sedentary time (P = 0.107), and MVPA (P = 0.963).
| Variables | Control | Intervention | P Valueb |
|---|---|---|---|
| Height, m | 1.73 ± 0.09 | 1.71 ± 0.09 | 0.575 |
| BW, kg | 69.57 ± 9.27 | 65.03 ± 8.2 | 0.216 |
| BMI, kg/m2 | 23.04 ± 1.94 | 22.11 ± 1.68 | 0.223 |
| % BF | 25.67 ± 10.66 | 24.46 ± 10.67 | 0.784 |
| LBM, kg | 49.7 ± 11.12 | 47.56 ± 10.53 | 0.634 |
| FM, kg | 16.7 ± 6.97 | 14.9 ± 5.72 | 0.494 |
Sample Characteristics at Enrollment by Groupa
4.1. Effects of One-Bout of High Intensity Resistance Training
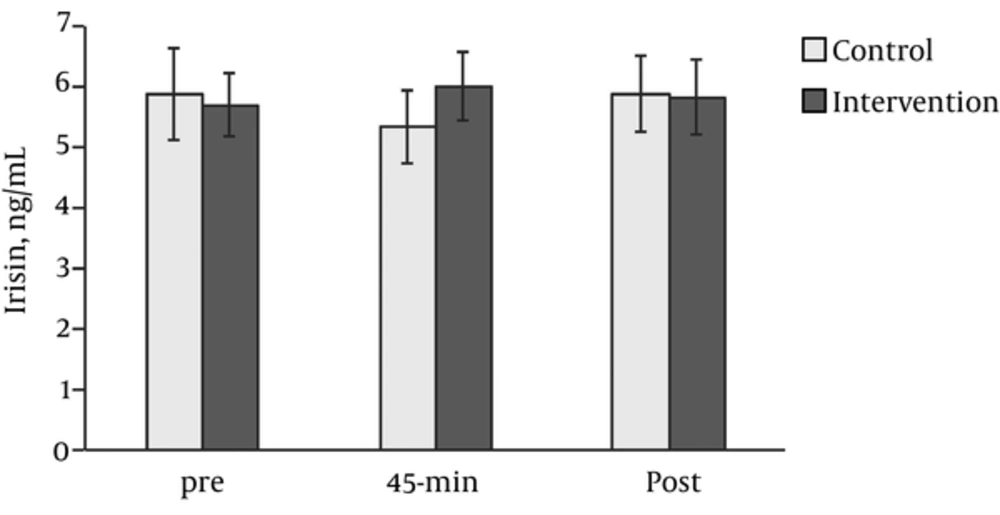
None of the ANOVA effects on serum irisin concentration were significant: group-time interaction (F (2,42) = 3.20, P = 0.051, ηG2 = 0.02), time (F (2,42) = 0.51, P = 0.606, ηG2 = 0.004), and group (F (1,21) = 0.14, P = 0.708, ηG2 = 0.004) (Figure 2). Only the control group showed statistically significant differences from 45 minutes to post-exercise (t (11) = 2.46, P = 0.023, d = 0.74) when pairwise comparisons were performed. However, the analysis of La levels before and after the acute session determined a statistically significant interaction effect (F (1,16) = 72.15, P < 0.001, ηG2 = 0.51), along with significant time (F (1,16) = 66.96, P < 0.001, ηG2 = 0.49), and group (F (1,16) = 52.61, P < 0.001, ηG2 = 0.51) main effects (Figure 2). Post-hoc analysis revealed very large differences in La induced by the intervention, t (11) = 11.79, P < 0.001, d = 3.55, and no difference for the controls (P = 0.828).
4.2. Effects of Three-Week High Intensity Resistance Training
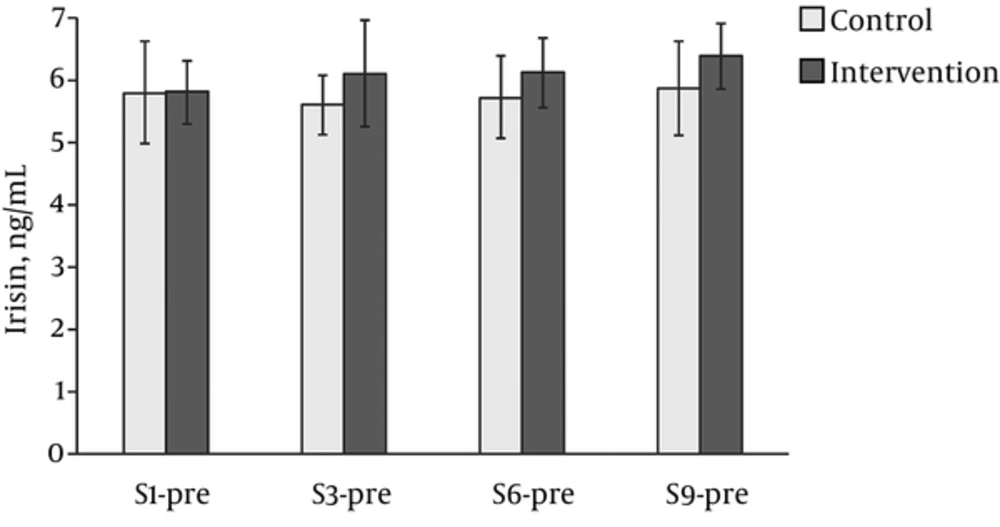
After the ANOVA results, no significant group-time interaction (F (1,22) =0.346, P = 0.792, ηG2 = 0.004), time (F (3,66) =0.484, P = 0.695, ηG2 = 0.01) and group (F (3,66) =1.258, P = 0.274, ηG2 = 0.01) effects were determined in serum irisin concentrations (Figure 3). In contrast, the ANOVA examining La levels pre-session 1 and post-sessions 1, 3, 6, and 9 of the three-week intervention study showed significant interaction (F (4,60) =35.68, P < 0.001, ηG2 = 0.33), time (F (4.60) =34.8, P < 0.001, ηG2 = 0.33), and intervention (F (1,60) =80.41, P < 0.001, ηG2 = 0.67) effects (Figure 3). Post-hoc analysis revealed that control group La levels did not change over time, while the intervention produced a statistically significant increase in La (P < 0.001), showing very large effect sizes from baseline to post-sessions 1, 3, 6, and 9 (d = 3.15, d = 3.57, d = 3.70, and d = 2.42, respectively).
Levels of (A) Irisin (ng/mL) and (B) Lactate (mmol/L) during 3 weeks of intervention. Pre - baseline, post- after session, S1 - session 1, S3 - session 3, S6 - session 6, S9 - session 9. Solid black - intervention group, solid grey - control group. Variables expressed in means and 95% confidence interval.
Additionally, as shown in Table 2, the analysis of body composition variables showed a statistically significant interaction effect on %BF, F (1,22) = 7.99, P = 0.010, ηG2 < 0.01. Post-hoc analysis showed statistically significant difference for the intervention group (t (13) =-3.37, P = 0.003) reflecting a large reduction (d = 0.93), despite the control group showing non statistically significant increase (d = 0.22). Similarly, pairwise comparisons revealed that while the control group showed no difference (P = 0.973, d = 0.01), intervention group largely increased (P = 0.016, d = 0.72) in LBM from pre to post.
| Variables | Control | Intervention | ANOVA 2 × 2 effects | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | G | T | G × T | |
| BW, kg | 69.57 ± 9.27 | 69.19 ± 9.29 | 65.03 ± 8.20 | 66.44 ± 6.54 | 0.288 | 0.414 | 0.158 |
| BMI, kg/m2 | 23.04 ± 1.94 | 22.92 ± 1.98 | 22.11 ± 1.68 | 22.62 ± 1.22 | 0.368 | 0.355 | 0.143 |
| %BF | 25.67 ± 10.66 | 25.90 ± 10.71 | 24.46 ± 10.67 | 23.51 ± 10.46 | 0.682 | 0.096 | 0.010 |
| LBM, kg | 49.70 ± 11.12 | 49.72 ± 11.10 | 47.56 ± 10.53 | 48.89 ± 10.88 | 0.742 | 0.088 | 0.097 |
| FM, kg | 16.70 ± 6.97 | 17.03 ± 7.09 | 14.90 ± 5.72 | 14.41 ± 5.52 | 0.766 | 0.881 | 0.471 |
| 1RMBP, kg | 59.38 ± 23.26 | 59.49 ± 24.46 | 59.38 ± 22.47 | 65.25 ± 26.14 | 0.766 | < 0.001 | < 0.001 |
| 1RMLP, kg | 149.89 ± 49.70 | 153.19 ± 51.42 | 145.85 ± 40.10 | 179.27 ± 41.94 | 0.555 | < 0.001 | < 0.001 |
| BP-to-BW | 0.84 ± 0.27 | 0.84 ± 0.26 | 0.90 ± 0.33 | 0.99 ± 0.36 | 0.701 | < 0.001 | < 0.001 |
| LP-to-BW | 2.14 ± 0.60 | 2.18 ± 0.59 | 2.22 ± 0.46 | 2.74 ± 0.47 | 0.137 | < 0.001 | < 0.001 |
All muscle strength variables showed significant interaction effects (Table 2): 1RMBP (F (1,22) =19.54, P < 0.001, ηG2 < 0.01); 1RMBP (F (1,22) = 20.84, P < 0.001, ηG2 = 0.03); BP-to-BW ratio (F (1,22) =18.93, P < 0.001, ηG2 = 0.01); and LP-to-BW ratio (F (1,22) =23.03, P < 0.001, ηG2 = 0.05). Subsequent post-hoc analysis determined no change for control group (1RMBP, P = 1.000, d = 0.00; 1RMLP, P = 0.504, d = 0.20; BP-to-BW ratio, P = 0.947, d = 0.02; and LP-to-BW ratio, P = 0.622, d = 0.15), while very large increase was observed for intervention group in all strength measures (1RMBP, P < 0.001, d = 1.81; 1RMLP, P < 0.001, d = 2.07; BP-to-BW ratio, P < 0.001, d = 1.76; and LP-to-BW ratio, P < 0.001, d = 2.11).
A descriptive analysis of intra-session changes over time, for sessions 1, 3, 6, and 9 shows variation in irisin in a group of 5 participants (Supplemetal file. Appendix 1).
4.3. Correlation Between Baseline Irisin Levels and Physical Fitness, Physical Activity and Diet
No statistically significant correlations were found between irisin levels (ng/mL) and physical fitness indicators at enrollment: BW (-0.29), BMI (0.05), %BF (0.10), LBM (-0.27), BP-to-BW (-0.07), LP-to-BW (0.30), and relative VO2max (rVO2max) (-0.19). Similar results were found between irisin levels and physical activity levels and diet: sedentary time (0.10), moderate to vigorous physical activity (MVPA) (-0.20); total energy intake (EI) (-0.28) proteins (-0.32), total fat (-0.21), carbohydrates (CHO) (-0.27), and added sugars (-0.23). However, the male subsample showed that irisin levels were significantly correlated with BW (-0.71, P = 0.005) and skeletal muscle mass (-0.67, P = 0.008).
5. Discussion
The major finding of the present randomized controlled trial was that irisin concentration did not change significantly during or after a single-bout, nor during three-week high-intensity resistance training compared to baseline for any of the groups of young individuals with healthy BMI. Although the results we obtained after one-bout of exercise are supported by previous studies (8-10, 18, 32), other studies have reported opposing results (3-5, 13, 16, 17, 20, 32-36). As supported by previous studies in adults, we did not detect significant changes in baseline irisin after a 3-week intervention (3, 8-11, 16, 34, 37). However, a few studies reported significant increases (14) or decreases (6, 7, 38) in baseline irisin concentrations, while results varied when middle-aged or older subjects were studied (7, 12, 14).
In regards to the experimetal design, only few studies analyzing exercise effects on irisin concentrations in adults utilized a control group simultaneous to the intervention group (4, 12, 14, 17, 22). The reported day-night variation of irisin is 74% in absence of exercise intervention (20). Therefore, it might be important to assess whether the behavior of irisin within the control group differed due to a temporal variation. Interestingly, one of these studies detected significant changes in irisin levels in the control group as reported in our study (14). Another study reported significant reductions in irisin levels (22), and the remaining studies with the control group reported significant changes only in the intervention group (4, 12, 17). As a result, we could not be certain whether some studies would have reached the same conclusions, or change the interpretation of their findings in the case that they included a control group (21).
In regard to the exercise program, prior investigators utilized different exercise modes obtaining different results, but there was a common ground on the majority that detected significant changes in the acute response of irisin to exercise: a, intensity (moderate-to-high); and b, mode (strength/power) (3, 7, 13, 14, 16, 33-36). These features are of similar characteristics to the exercise prescribed in the present study. Therefore, it was critical to ensure the achievement of the targeted intensity. In this sense, we confirmed significant changes on La levels at session 1 (one-bout intervention) and through the 3-week exercise intervention.
In terms of effectiveness, the three-week resistance training program, used for the present investigation resulted in significant improvement in body composition (%BF and LBM), and muscle strength (1RMBP, 1RMLB, BP-to-BW, and LP-to-BW) outcomes. The %BF decreased by a 4.13%, and LBM gain accounted for an average of 1.33 kg in only a three-week period. Significant strength gains in the intervention group ammounted to 10.2% in upper extremities and 24.9% in lower extremities. However, no related changes in serum irisin concentrations were observed. The present results seem to be consistent with other previous investigations that did not find any effect of irisin concentrations on strength improvements (7, 11), resting metabolic rate increases (14), or body composition improvements (7). On the other hand, the work carried out by Kim et al. (22) showed that chronic resistance training might be an efficient intervention method to increase irisin levels and prevent decline in muscle function in old athletes (> 65 years old). On those lines, Huh et al. (34) found increases in serum irisin levels after performing one whole-body vibration exercise session. Moiernneia et al. (6) reported no changes after performing an acute bout of low or high intensity resistance training, but serum irisin was significantly decreased after eight weeks. These results are difficult to interpret because no changes in performance, or body composition were observed (6). In summary, results reported in the literature vary greatly, might be caused by the difference in the training protocols, exercise modes, intensity, equipment (i.e. vibration, free weights, machines, etc.), and initial physical fitness level making it difficult to interpret the acute and long-term effects of resisntance training on irisin.
In the present study no positive or negative associations between baseline physical fitness, physical activity, or diet and serum irisin concentrations were found when both males and famales are pooled together. However, when analyzing males, irisin concentrations were negatively correlated to BW and skeletal muscle mass. Our results are consistent with Moreno et al. (18), who reported similar results in BW in a sample of 428 adults. Some cross-sectional studies found negative association between irisin and cardiorespiratory fitness (VO2max or peak, respiratory quotient, or oxygen pulse) in healthy individuals (9, 13). In contrast, Lecker et al. reported positive association in heart failure patients (39). The authors found that a group of heart failure patients with high aerobic performance, showed higher mRNA levels of PGC-1α and precursor irisin gene. This association could explain the positive relationship between VO2max and circulating irisin concentrations reported by other authors (39). Some studies with similar sample size showed positive associations between irisin concentrations and BMI (9, 16), and muscle mass and strength (16, 32). These varied results may be related to the heterogeneous type of sample (i.e. healthy, disease, young, elder, etc.) which is linked to a specific physical fitness profile. Therefore, considering that we were only able to detect associations between irisin levels and muscle mass in males, but not strength-related variables, this association will still remain unclear.
Results of the present investigation support the idea that exercise-induced changes in circulating irisin may need further investigation. Some studies have reported increases during exercise (4), inmediately post-exercise (6, 17, 36), and others found them after one hour following exercise (5, 17). Therefore, time of sampling might be a key factor when analyzing the acute response of irisin to exercise (5, 17, 36). On another note, methods utilized to quantify irisin have brought controversy to their questioned ability to detect irisin concentrations in serum and plasma. Bostrom et al. (1) first described irisin as a cleaved and secreted part of FNDC5. Their findings are under debate due to: a, a mutation on the start codon of human FNDC5 gene discovered in 2013 (2); b, the polyclonal antibody (pAb) used at the initial study in 2012 (1) was not expected to bind to the cleaved irisin protein (40). A more recent article found discrepancies in the detection and quantification of circulating irisin when comparing different irisin kits (40). Their results showed no correlations when comparing concentrations obtained using different ELISA kits and those obtained using western blot and mass spectrometry. They concluded that some ELISA kits had a high-cross reactivity on complex samples such as serum or plasma (40). In this line, another recent study published by Jedrychowski et al. (41) reported opposed results. They showed that human irisin was mainly translated from its non-canonical start codon, detected in blood plasma, and regulated by exercise. Furthermore, they reported that Albrecht’s study had several serious methodological deficiencies, and confirmed that the ELISA kit utilized in this study should be able to detect irisin if present in the biological sample when utilizing tandem mass spectroscopy (41).
The design, inclusion criteria, and blocking randomization guaranteed that both control and intervention groups were equivalent and homogeneous at the beginning of the study. Some possible confounding variables were controlled by matching subjects by age, sex, BMI and LBM prior to randomization into control and intervention group. Additionally, the inclusion of the control group and the very well designed and supervised exercise intervention strengthen the obtained results. This enabled us: a, to address the problem related to day-night fluctuation of irisin throughout the duration of the study; and b, to confirm that the exercise program was intense enough to induce physiological adaptations in the exercise group.
This study is not without limitations. The unexpectedly large attrition rate of 35% (see Figure 1) made it difficult to achieve the desired statistical power of 80%. However, we were able to estimate and report the standardized effect sizes for each comparison of interest. One major problem was that the biochemical and biological properties of irisin and its quantification remain largely debated. Another possible limitation might have been that FNDC5/irisin gene expression is regulated differently depending on diet, hormonal conditions and differ among organs (brain, adipose tissue, and muscle), suggesting that circulating irisin might be the sum of irisin produced by different organs and tissues (15). Although we did not intervene in diet, participants were requested not to modify their dietary habits. In this regard, analyses showed no differences in physical activity, physical fitness, or diet between control and intervention group at the beginning of the study (14, 22). Finally, although we did not examine the effect of cold exposure (42), and control and intervention subjects were under the same conditions (72°F, 22°C), we do not know to what extent the temperature might have different effects in the subjects.
The major finding of the present randomized controlled trial was that irisin concentration did not change significantly during or after a single-bout, nor during 3-week high-intensity resistance training when compared to matched controls. Interestingly, only irisin levels in the control group were significantly increased. In addition, no significant associations were found between irisin levels and physical activity, diet, or physical fitness, except baseline serum irisin levels, BW and skeletal muscle mass in males. Results derived from this study demonstrate that research efforts should focus on studying the effects of exercise in irisin using different exercise modes in the same experiment with more than one group including a control group. These studies should control for diet and hormonal status to detect possible confounding variables, and analyze other associated markers to FNDC5 expression in diverse tissues (muscle and fat depots) in addition to blood samples. Finally, research should cocentrate on improving the understanding of the biochemical and biological properties, and regulation of irisin in humans, and continue to develop valid and reliable methods to detect and quantify irisin.