1. Background
Osteomyelitis is an inflammatory process of bone with resultant destructive and necrotic change accompanied by new bone formation, which can progress to a chronic or persistent stage (1). The incidence of osteomyelitis differs according to the primary inoculation and the underlying disease with a range of 1% to 15% (2, 3). Rubin evaluated 1,351,362 non-obstetric hospital discharges in New York city hospitals in 1995 and showed that an estimated 2000 (0.00147) were patients with S. aureus osteomyelitis with an average hospital stay estimated to be 23.9 days with subsequent incurred costs of 35000 dollars indicating a significant burden to the health economy (4). S. aureus is the most common pathogen responsible for both acute and chronic forms of osteomyelitis (1, 5, 6). S. aureus has developed a plethora of strategies to evade the host innate and adaptive immune systems, with a high resistance to different therapeutic options (7). Tice et al. in a long-term follow-up study of osteomyelitis treated in the outpatient setting found that 31% of 452 patients had recurrent infection, most within one year of occurrence (8).
Stengel et al. in a systematic review with meta-analysis of antibiotic therapy for osteomyelitis reported that the penetration of an antibiotic agent into an infected bone is determined by the pharmacological characteristics of the drug, degree of vascularization, soft-tissue coverage and the presence of a foreign body (9). To overcome the limited penetration of the systemic agents into poorly vascularized bone, systems for local delivery of antibiotics have been implemented in the previous studies (10, 11). Bucholz and Engelbrecht introduced the delivery of high-dose antibiotics to the skeletal tissues by resin (Palacos) in 1970 (12). Bone cement (13, 14) and polymethylmethacrylate (PMMA) beads (15) are other skeletal drug-delivery systems commonly used for this purpose. However, all of these techniques are static in nature with the potential of bacterial resistance.
Probiotics are viable cell preparations that have beneficial effects (16). Different strains of probiotics have different strategies to compete with S. aureus. Probiotic has been used in the treatment of many clinical infections in the middle ear, bladder, gut and the vagina of the humans and in the wounds of the animals (17-20). Since some probiotics are a dynamic source of antimicrobial agent with variable strategy, we hypothesized that specific probiotic strains of lactobacilli with predefined anti-infective properties against S. aureus could inhibit S. aureus-induced osteomyelitis. We, therefore, implemented an infection model following trauma fixation and investigated the therapeutic potential of the probiotics. A successful therapeutic strategy at this stage of trauma comprising an infected fracture with metalwork in situ would prevent the further development of infection in critically ill patients and allow the retention of fixation until osseous union occurs.
2. Methods
2.1. Study Design
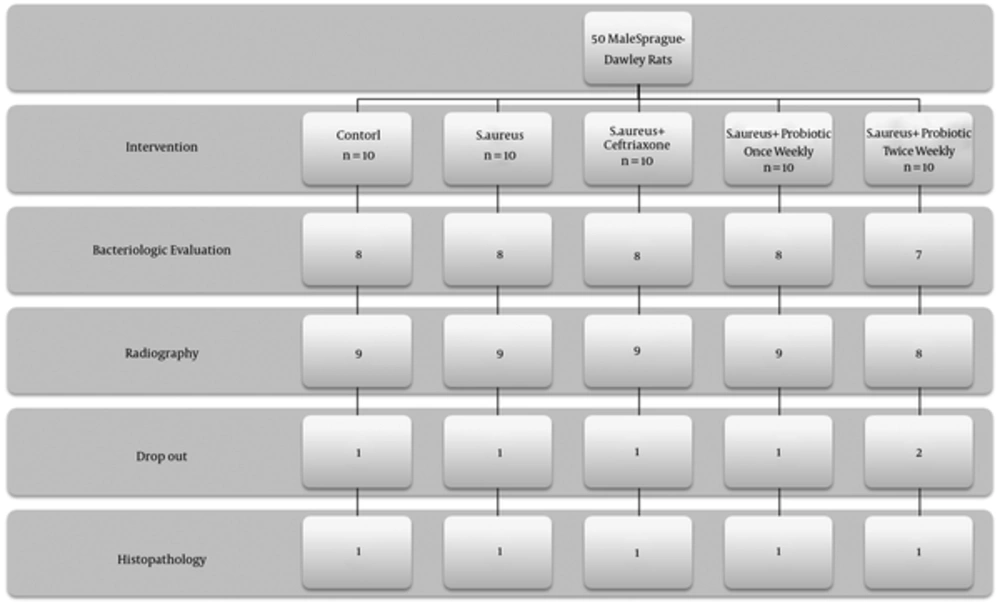
Fifty male Sprague-Dawley rats were assigned to five groups:
Control (pinned femur fracture + 0.1 mL Phosphate buffered saline (PBS))
S. aureus (pinned femur fracture + 0.1 mL of S. aureus)
S. aureus +ceftriaxone (pinned femur fracture + 0.1 mL of S. aureus + ceftriaxone (50 mg/kg) every 24 hours for 3 weeks)
S. aureus + once weekly probiotic (pinned femur fracture + 0.1 mL of S. aureus + 0.1 mL Lactobacillus (LAD) every week for three weeks)
S. aureus + twice weekly probiotic (pinned femur fracture + 0.1 mL of S. aureus + 0.1 mL LAD twice weekly for three weeks)
The rats were humanely euthanized three weeks after the surgery with a lethal dose of Xylazine. The development of osteomyelitis was documented using radiographs (all animals), postmortem microbiological (n = 8 per group) and histopathological (n = 1 per group) analysis. The study protocol was approved by the **** animal care and use committee (date of approval: 29.1.2010, Code number: 16).
2.2. Bacterial Preparation
2.2.1. Bacterial Strains
Eight strains of LAD and five strains of staphylococcus were purchased from the Iranian research organization for science and technology (IROST). These strains were selected due to their potential effect against S. aureus in the previous studies (18, 21-25).
2.2.2. Antimicrobial Activity
The antibiotic susceptibility of all isolated strains was tested based on the standard methodology recommended by the Canadian committee on antibiotic resistance (26). A modified Coconnier model (27) was used to assess the antibacterial activity of each LAD with a modified “spot on the lawn” method described by Navarro (28). Based on the antibacterial activity of each LAD, Lactobacillus casei subsp. casei (ATCC: 39392) was the most potent probiotic against S. aureus, subsp. aureus (ATCC: 33591) and was selected for the rest of the study.
2.3. Surgical Procedure
The surgical procedure was a modification of that outlined in detail by Skott et al. (29). Each rat was anesthetized using intraperitoneal injections of ketamine (100 - 200 mg/kg) and xylazine (2.0 - 4.0 mg/kg). The right femur was prepared aseptically and a 20-gauge needle was used to create an entry port into the distal aspect of the medullary canal of the femur which was reamed to allow the insertion of the intramedullary pin. An inoculation dose of 0.1 mL of staphylococcus suspension (106 bacteria) was slowly injected into the medullary cavity in the experimental groups, and in the control group PBS was injected instead.
A sterile stainless steel Kirschner wire, 1 mm in diameter and 20 mm in length was then inserted into the medullary canal and seated in the cortical bone in the proximal aspect of the femur. Thereafter, a mid-shaft closed fracture of the right femur was created using a specifically designed fracture apparatus that consisted of a blade which was placed in contact with the femur to fracture the femoral cortex before rotating the femur and fracturing the remainder of the cortex.
2.4. Treatment Procedure
We defined two time intervals for the probiotic injection to assess the dose-response effect. Rats in the S. aureus + probiotic group received 0.1 mL Lactobacillus casei subsp. casei (106 CFU in 0.1 mL) once or twice weekly starting 24 hours after the inoculation, via two sub-periosteal injections near the fracture site for the duration of the study (three weeks). The S. aureus + ceftriaxone group received ceftriaxone (50 mg/kg) every 24 hours, starting 4 hours after the inoculation, via a subcutaneous injection for the duration of the study.
2.5. Radiographic Assessment of Osteomyelitis
Lateral radiographs of the right hind limb were obtained at weeks one, two, and three postoperatively. Two individuals (BS, CH) evaluated the radiographs focusing on three regions of interest (RoI):
(1) Proximal metaphyseal area
(2) Diaphyseal region involving the site of the fracture
(3) Distal metaphyseal area
Each radiograph was assessed based on a validated system used by Lucke et al. (30, 31). The following radiographic changes were estimated for each ROI: (1) osteolysis, (2) soft tissue swelling, (3) periosteal reaction, (4) general impression and (5) deformity. The changes were given a score corresponding to the following scale: 0 - absent, 1 - mild, 2 - moderate, or 3 - severe. For the general impression evaluation, a 0 represented a normal appearing femur/fracture and 3 represented severe changes present overall. In addition, sequestra formation (6) and spontaneous fracture (7) were evaluated for each femur as a whole and were awarded a score of 0 - absent or 1 - present. The scores were then summated with the highest possible total score being 47. The mean scores from the two evaluators were used for the statistical evaluation.
2.6. Recovery of Bacteria
Three weeks after the surgery, the femora of the right hind legs were dissected free from the underlying tissue under sterile conditions. The K-wires were aseptically retrieved from the operated femora prior to the snap freezing. Under sterile conditions, the K-wires were placed in 2.0 mL of sterile PBS, then spun for five minutes and centrifuged (10,000 RPM for 10 seconds) to dislodge adherent bacteria. A hundred micro liter samples were then collected for the microtiter dilution and the results were used to calculate the CFU/pin.
Each femur was snap frozen and ground to powder under sterile conditions. A hundred and fifty milligrams of the bone powder was agitated in 1.5 mL of sterile PBS for 2 minutes by vortex (3000 RPM) and the suspension was centrifuged for 10 seconds (10,000 RPM). A hundred microliters of the supernatant was withdrawn for serial dilution and sampled for microtiter dilutions to calculate the CFU/femur.
2.7. Microtiter Dilution and Viable Bacterial Counts
The CFU was determined in quadruplicate by collecting an aseptic sample. Tenfold dilutions were made (10 - 1 to 10 - 2) using PBS. Twenty microliters was streaked across a Trypticase Soy Agar (TSA) (Beckton Dickinson Diagnostic Systems, Sparks, MD) plate. The plates were then incubated aerobically at 37°C for 24 hours at which time the number of colonies was counted. Dilutions with up to 30 colonies present were used to calculate the median CFU/pin or CFU/femur. A sample was taken from each media plate to perform DNase, coagulase, catalase and novobiosin tests, in addition to culturing it in specific media to verify the specificity of colonies similar in shape and color.
2.8. Histopathologic Evaluation
The operated femur from one rat in each group was used for histopathologic evaluation. The sections were evaluated by a clinical pathologist who was blinded to the treatment group. Slices of the same osseous plane were evaluated based on the following parameters of infection in accordance with Petty and coworkers (32): 1) abscess formation, 2) sequestrum formation, 3) cortical enlargement, 4) cortical destruction, 5) general impression.
2.9. Statistical Analysis
Based on the results of Lucke’s study (30), difference and standard deviation of 50000 and 80000 CFU per gram of bone were assumed for calculating sample size, respectively. In a one-way analysis of variance (ANOVA) study, sample sizes of eight animals in each group achieved 81% power to detect the differences among the means versus the alternative of equal means using an F test with a 0.05 significance level (33).
Kolmogorov-Smirnov test was used to determine if the data exhibited a normal distribution. The radiographs were scored by two independent orthopaedic surgeons anonymously. Intraclass correlation coefficients (ICC) were calculated to check the inter-observer reliability. The radiographic results were averaged and compared using the repeated measure ANOVA. If there was a missing follow-up, its value was calculated from the mean of nearby points. The recovered CFU/pin and CFU/femur were reported as mean CFU and were compared across all five groups using the ANOVA, with significance set at P < 0.05. The distributions of CFU/pin or CFU/femur were compared for each pair of treatment groups using the Scheffe post-hoc test with significance set at P < 0.05. All the analyses were performed using the IBM SPSS Statistics Standard 20.0 (Chicago, IL, USA).
3. Results
3.1. Radiographic Assessment
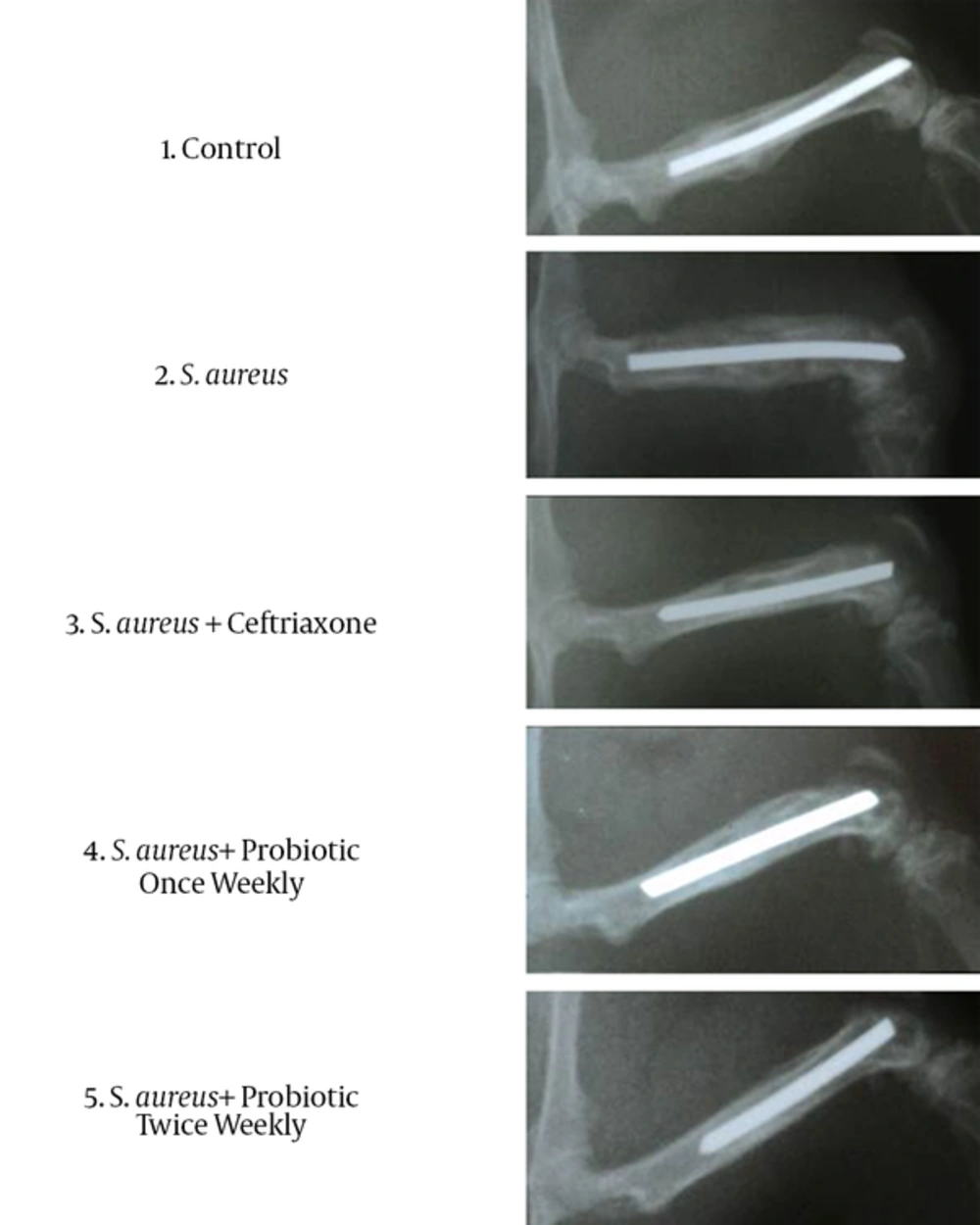
At the third week postoperatively, the fracture was most evident in the S. aureus group, followed by the S. aureus + ceftriaxone group, but was only weakly evident in the control and probiotic groups. The radiographs from the control group were characterized by callus formation limited to the fracture site. There was also a periosteal reaction in the control group accompanying the newly formed bone which represented the continuation of the healing process. The S. aureus group was characterized by extensive periosteal reaction extending throughout the femoral length. New bone formation beside the periosteal reaction in the S. aureus group did not bridge the fracture site and was accompanied by osteolysis along the femur. The S. aureus + ceftriaxone group was characterized by limited periosteal reaction along with new bone formation which bridged the fracture site. The S. aureus + Probiotic once weekly group was characterized by mild periosteal reaction with new bone formation that bridged the fracture site. There was no evidence of osteolysis in this group. The S. aureus + Probiotic twice weekly group was characterized by minor periosteal reaction and cortical thickening without any evidence of a fracture line (Figure 2).
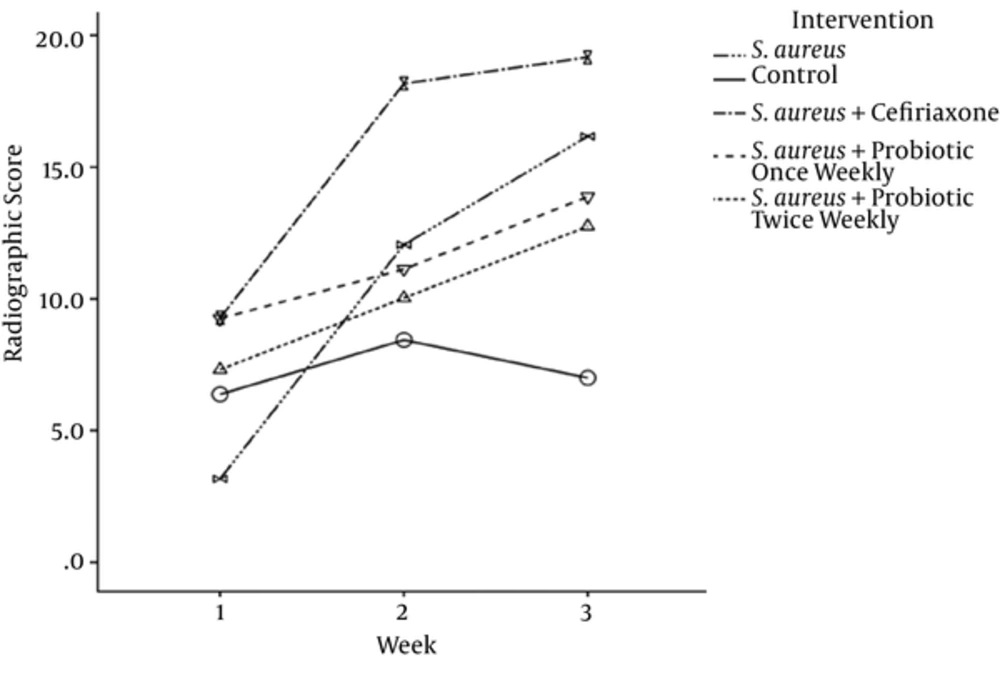
Intra-class correlation coefficients (ICC) for the inter-observer reliability of the radiographic scores in weeks one, two and three were 0.52, 0.45 and 0.59, respectively. The Kolmogorov-Smirnov test revealed a normal distribution of this variable (P = 0.2). The radiographic scores at week one were similar in all of the five treatment groups (ANOVA, P = 0.62). The repeated measure ANOVA to test the radiographic scores during the follow-up time between the intervention groups revealed no significant differences throughout the follow-up period (P = 0.179) (Figure 3).
3.2. Recovery of Bacteria
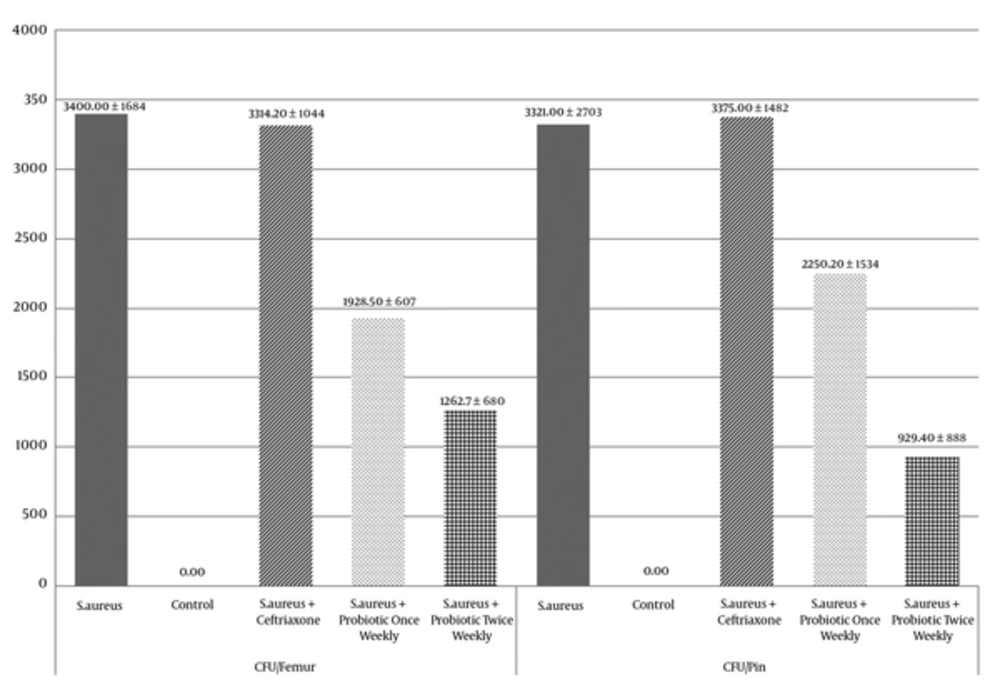
No bacteria were recovered from rats in the control group. The ANOVA revealed a significant difference in the CFU/femur (P < 0.001) and CFU/pin (P = 0.001) across all five treatment groups. When the results of each arm of the study were compared pair-wise using the Scheffe post-hoc test, the CFU/femur was significantly lower in the S. aureus + Probiotic twice weekly group in comparison with the S. aureus (P = 0.008) and the S. aureus + ceftriaxone (P = 0.012) groups; the CFU/femur was not significantly different between the S. aureus group compared with the S. aureus + ceftriaxone group (P = 1.0) and the S. aureus + Probiotic twice weekly group compared with the S. aureus + Probiotic once weekly group (P = 0.8). The CFU/pin results followed a similar pattern to CFU/femur. (Figure 4, Table 1)
| Control | S. aureus | S. aureus + Ceftriaxone | S. aureus + Once Weekly Probiotic | S. aureus + Twice Weekly Probiotic | |
|---|---|---|---|---|---|
| CFU/Bone | |||||
| Control | - | < 0.01* | < 0.01* | 0.02* | 0.23 |
| S. aureus | < 0.01* | - | 1.00 | 0.12 | < 0.01* |
| S. aureus +ceftriaxone | < 0.01* | 1.00 | - | 0.16 | 0.01* |
| S. aureus + once weekly probiotic | 0.02* | 0.12 | 0.16 | - | 0.80 |
| S. aureus + twice weekly probiotic | 0.23 | < 0.01* | 0.01* | 0.8 | - |
| CFU/Pin | |||||
| Control | - | 0.01* | < 0.01* | 0.16 | 0.87 |
| S. aureus | 0.01* | - | 1.00 | 0.80 | 0.12 |
| S. aureus +ceftriaxone | < 0.01* | 1.00 | - | 0.75 | 0.09 |
| S. aureus + once weekly probiotic | 0.16 | 0.80 | 0.75 | - | 0.66 |
| S. aureus + twice weekly probiotic | 0.87 | 0.12 | 0.09 | 0.66 | - |
3.3. Histopathology
The specimen from the control group was characterized by the presence of callus. In contrast, the specimen from the S. aureus group was characterized by active reactive bone and osteoid formation with fibrosis, extending to the marrow space and the cortex with encasement of callus including hyaline cartilage. Acute suppurative inflammation was seen which was enclosed in the reactive changes. The specimen from the S. aureus + Ceftriaxone group was characterized by the presence of severe and acute suppurative inflammation plus bone sequestrum (acute suppurative osteomyelitis) extending to the fracture site and the periosteal surface. There was a narrow rim of callus formation that was composed of hyaline cartilage at the periphery of the inflammation. The specimens from Probiotic groups were similar to that of S. aureus group except for more prominent fracture site callus formation in the probiotic once weekly group, and prominent bone sequestrum and infarct in the probiotic twice weekly group.
4. Discussion
The results of the present study revealed that viable Lactobacillus casei can inhibit acute infection caused by S. aureus. Although the histopathological deduction is limited due to inadequate histologic specimens, this assessment revealed that probiotic bacteria may slow down the healing process, in spite of improving the bacteriologic results. We could not deduct on the radiographic assessment due to the lack of significant difference between the intervention groups. This may be due to limited sample size or low inter-observer ICC. To our knowledge, this is the first study to demonstrate the administration of a viable probiotic strain through a parenteral route (subperiosteal) in the in vivo treatment of osteomyelitis.
Hospitals in the Europe link for infection control through surveillance (HELICS) have reported that 48.6% of surgical site infection secondary to orthopedic surgery was due to S. aureus (34). S. aureus adheres to fixation devices by producing a glycocalyx biofilm around prosthetic materials and forms large micro-colonies. The biofilm confers bacterial resistance to antibiotics and ingestion by neutrophils (6). Conversely, bone necrosis developing early in the disease process limits the possibility of eradicating the pathogen and leads to chronicity (35). Treatment strategies for osteomyelitis mainly consist of three stages, firstly antimicrobial agents, secondly surgical techniques and thirdly amputation (36). A variety of antimicrobial agents with a different spectrum of action, pharmacokinetics and pharmacodynamics have been used in this manner. Due to the physiological and anatomic characteristics of the bone, antimicrobial therapies are thus suboptimal in the treatment of osteomyelitis. Surgical techniques, including limb salvage, muscle grafts, Ilizarov techniques, antibiotic coated prostheses and antibiotic bone cement have also been applied with varying degrees of success (1, 36).
The definition of a Probiotic is: a live micro-organism which when administered in adequate amounts confers a health benefit to the host (FAO/WHO report, October, 2001). There are few studies that have assessed the parenteral administration of the probiotic. Sheil studied the parenteral administration of viable probiotic to alleviate mice induced colitis and arthritis (37). L. casei is a gram positive bacillus that ferments carbohydrates mainly to lactic acid which consequently leads to environmental acidification down to a pH of 3.5 (38). There are several hypotheses regarding the mechanism involved in the inhibition process of S. aureus by L. casei. They include environmental acidification, production of hydrogen peroxide, production of bacteriocins and nutritional competition (38). Sadowska et al. showed that LAB could inhibit staphylococcal adhesion and slime layer production using bacteriocin and bacteriocin-like inhibitory substances (24). In addition, laboratory studies revealed that the acidification of a growth medium by addition of lactic acid down to pH 4.5, completely inhibits the growth of S. aureus (38). The ratio of S. aureus against LAB is another determinant of the growth inhibition. Therefore, in media where the S. aureus population is larger than LAB, the size of the S. aureus can reach 1010 CFU/mL (a level at which S. aureus begins enterotoxin production and limits its growth). However, for a S. aureus: LAB ratio of 1/1 and 1/10, maximum population reached by S. aureus is about 106 and 105 CFU/mL, respectively (38).
Lactic acid bacteria are generally safe with limited clinical infections reported in the humans where LAB were the causative pathogen (39) and few case reports describe the isolation of LAB from patients with infective endocarditis, localized infection and septicemia (40, 41). Adams and Marteau (42) concluded that LAB posed no significant risk of infection if administered orally. There are few studies on the parenteral use of probiotic (26, 37, 38, 43). Therefore, extending LAB safety to parenteral administration needs further study.
Our study has some limitations. Our experimental model does not exactly mirror the clinical scenario. Therefore, we cannot conclude that probiotic could be a treatment option for implant-related osteomyelitis. Meanwhile, this can be a new branch for potential treatment option in the future. Due to an executory problem in the radiology department, we could not radiograph one group (S. aureus + Probiotic twice weekly) in week 2 and, therefore, inputted the missing data by averaging the radiographs in weeks 1 and 3. We also could not determine the exact mechanism of the inhibition of S. aureus by L. casei in vivo and vitro. In the bacteriologic evaluation, we used selective media for S. aureus where L. casei could not grow and confound the finding. In addition, we performed specificity tests to validate observed colonies as S. aureus and differentiate them from other similar colonies such as Streptococcus and other strains of Staphylococcus. In the histologic evaluation, the number of specimen from each intervention group was not adequate for statistical analysis. Therefore, we narratively described the histologic finding without statistical analysis.
In conclusion, parenteral administration of viable L. casei inhibits S. aureus-induced osteomyelitis as shown by bacteriologic analysis, but makes no difference to radiologic union rates. This could be the first step in developing an effective, safe and inexpensive treatment for osteomyelitis.