1. Background
The immunity system uses the antioxidant defense system, which includes antioxidant enzymes (catalase, glutathione peroxidase, and superoxide dismutase) and non-antioxidant enzymes (including vitamins A, C, and E) to restrict the negative effects of free radicals (1). Catalase enzyme is iron linked and is mainly located in organelles called peroxisomes. It converts H2O2 to H2O, and its activity is similar to that of superoxide dismutase (SOD), its highest activity is in the liver, and its lowest is in the skeletal muscle (2).
The increase of free radical production during intense exercise can cause inflammation and serious injuries to athletes. With the start of tournament season and intense training for the preparation of athletes and lack of adequate recycling in exercises, the immunity system of the athletes may be weaken and could not deal with oxidants and the progenitor factors even if they do not cause much harm to the athletes (3). During and after the exercise, oxidative stress occurs only if the exercise-induced ROS production exceeds the body's antioxidant defense potential. It seems that antioxidative balance between physical and oxidative stress plays a decisive role in the occurrence of this phenomenon (4).
Antioxidant enzymes superoxide dismutase, glutathione peroxidase, catalase, and antioxidant substances are among the antioxidative materials of the antioxidative system of the body that has an inherent place within this system (5). Because of compatibility with exercise training, unlike oxidative stress, it causes a decrease in the production of free radicals and increases the production of free radicals (6). The finding from various physical exercises on the increase or decrease of the free radicals raises a fundamental question about the types of training. As physical activity increases oxygen consumption, free radical production increases as well. It should be determined whether performing physical activity, due to its free radical production, is beneficial or harmful for the athletes. Some of the physiological and functional effects of tapering with high intensity and low volume, low-intensity and moderate volume, and rest only have been investigated in a semilong distance marathon (7).
Among the total blood volume, red blood cell volume, citrate synthase activity, muscle glycogen concentration, muscle strength, and run time to exhaustion, only tapering high intensity, and low volume reached the optimal level (7). Many studies have shown that a decrease in the volume of exercise training (50% - 70%) is a proper method for preserving compatibility in runners (8) and well-trained cyclists (9). Football is considered a combined activity. Combined activities are activities that include aerobic and anaerobic metabolism. Thus, the players will undergo physiological pressures during both preparations and competition (10). The awareness of coaches and players about these pressures could be helpful in designing an optimal practice and improving players’ performance. Football is a sport of high intensity interval in which players suffer from high physical pressure in a relatively long period (90 minutes including two 45-minute halves with a 15-minute break).
Ninety percent of the need in a footfall match is provided through the aerobic system, and football players run 10 km in an intensity near the anaerobic threshold (11). Aguiar et al. (2005) and Aguilo et al. (2005) conducted a study to prove the occurrence of oxidative stress during exhaustive exercise and measured antioxidant responses (11, 12). Nevertheless, there is little information available about oxidative stress of a football match on players, especially the female ones. The present study aimed at determining the response markers of oxidative stress on female footballers. What accounts for this study is the function and responsibility of the 2 enzymes: glutathione reductase and catalase which are essential for many physiological responses of the body (7).
Only a few studies have examined the antioxidant enzyme in red blood cells, particularly in female football players. Conducting the research in real terms, the use of the female participants, using a drill with acceptable international standards, and comparing it with the real competition were among the reasons for the innovation in the design of the present study. Several factors have made this study important and they are as follow: lack of such studies in the country and the ambiguity in the condition of glutathione reductase and catalase after different training methods, especially in females’ sports. Little research has been conducted on the following subjects: the antioxidant and biological functions of glutathione reductase and catalase in humans at a real state, the therapeutic effect of exercise (especially interval training) for diseases caused by interval training, and the concentration of glutathione reductase and catalase in female football players. The reduced catalase activity in erythrocytes is also associated with an increase in some cardiovascular risk factors. Hence, this research was designed to investigate and compare the effects of an interval training session on the concentration of glutathione reductase and catalase activity in female football players.
2. Methods
In the present study, 147 female football players were selected from 7 teams with 5 years of experience. To control the menstrual cycle, participants were selected through interviews from different teams. None of the participants had symptoms of iron deficiency anemia, and they were all in perfect health. Their body fat percentage, BMI, height, weight, and VO2max, were measured. Then they were divided into 2 groups of experimental (N = 22) and control (n = 22) (Table 1).
Participants' features were measured 20 hours before the start of the study (Table 1). Skinfold thickness was measured to the nearest 0.5 mm at the abdominal, triceps, and supra iliac sites using an Eiken skinfold caliper (MK-60, Yagami, Japan). The body fat percentage of the participants was measured by the following formula:
% Body Fat = (0.41563 x sum of skinfolds) - (0.00112 x square of the sum of skinfolds) + (0.03661 x age) + 4.03653.
To assess VO2max, the participants were asked to complete a continuous incremental exercise test to voluntary exhaustion on a calibrated treadmill according to Bruce protocol (RodbyTM, RL 1600E, Enhorna, Sweden).
The participants were in preseason tournament and Copenhagen football test. Copenhagen football test consists of two 45-minute periods with a 15- minute break between them, which was designed according to the pattern of football match (13). This test was used to simulate a football game after continuous team training and all players were in the preparation stage, while the control group only performed their normal activities. During the study, participants were asked not to alter their diet and register the food items consumed in the 3 days prior to the test. On the final assessment day, the participants who were in the fasting state and had not participated in sports activities in the last 48 hours were sent to the laboratory.
The first blood sample was taken from the brachial vein and the second blood sample was collected immediately after Copenhagen test in the experimental group. The experimental protocol was approved by the research ethics committee at Islamic Azad University, Mahallat Branch, Arak, Iran. They expressed their appreciation to the colleagues in the field. In addition, no conflict of interest was declared by the authors. This article was derived from a master's degree thesis conducted in 2015, entitled: “The effects of copenhagen football test on glutathione reductase and catalase activity in female football players” with the code 20021404931002. This study has been financially supported by the research department of Islamic Azad University, Mahallat branch.
To measure the catalase activity, 9 mL of blood were collected and then 3 mL were collected in tubes containing sodium citrate. Three mL vial containing EDTA to measure hemoglobin and glutathione reductase and 3 mL were dropped in tubes without anticoagulant.
Then using centrifuges, blood samples were separated from erythrocytes and were stored at -80°C. Glutathione reductase and catalase enzymes were studied in the laboratory by human kit, using ELISA. Glutathione reductase was measured using a special laboratory kit (ELISA (R and D) System, Minneapolis, MN, OD scale or light meter from Med system Bender factory made in Austria) according to the protocol provided in the kit. The sensitivity of the kits was 3.2 pg.mL-1. After the EDTA blood samples were obtained and washed by adding an equal volume of deionized distilled water, hemolytic 50% were prepared, and 500 mL of this hemolytic were stored at -80°C for measuring enzyme activity of glutathione reductase. Blood samples were taken on sodium citrate, washed, and prepared for hemolytic by adding phosphate buffer for dilution 1.500 and were tested immediately as duplicate. The enzyme activity of glutathione reductase was reported and calculated in U/gHb. Catalase activity in each sample was calculated by measuring primarily hydrogen peroxide decomposition reaction at a constant rate and the k.g Hb-1 ratio. Catalase and glutathione reductase enzyme activity measurement were carried out using a double deem spectrophotometer model Cencil-9000. In the present study, SPSS Version 21 software and nutrition food processor were used for data analysis. All descriptive statistics are shown as average and standard deviation from the average. Paired t-test and analysis of covariance were used to compare the results of the pretest and posttest and evaluate the effectiveness of the interventions. The significant level was set at P < 0.05.
3. Results
Personal characteristics such as age, weight, height, body mass index, body fat percentage, and degree of cardio respiratory fitness (maximum oxygen consumption) of the participants were presented in Table 1. No significant difference was observed in terms of objective variables between the 2 groups (P < 0.05) (Table 1).
| Variable | Exercise Group (n = 22) | Control Group (n = 22) |
|---|---|---|
| Age | 96.4 ± 08.28 | 20.5 ± 27.27 |
| Height, cm | 90.7 ± 85.161 | 98.5 ± 09.161 |
| Weight, kg | 02.16 ± 67.59 | 83.10 ± 69.61 |
| Body Fat, % | 26.7 ± 36.26 | 56.1 ± 29.25 |
| VO2max, ml/kg/min | 36.7 ± 22.48 | 84.6 ± 35.46 |
| Body mass index, kg/m2 | 86.2 ± 77.22 | 66.2 ± 78.23 |
aValues are expressed as mean ± SD.
No significant difference was found in the amount of calories, protein, carbohydrates, and fats between the 2 groups in the 3 days before the test (P < 0.05) (Table 2).
| Variable | Exercise Group (n = 22) | Control Group (n = 22) | P Value |
|---|---|---|---|
| Calories, MJ per deciliter | 2734.7 ± 390.8 | 2360.2 ± 258.7 | 0.78 |
| Protein, % of energy | 106.27 ± 20.2 | 96.34 ± 17.4 | 0.69 |
| Carbohydrates, % of energy | 391.5 ± 95.2 | 338.58 ± 99.3 | 0.57 |
| Fat, % of energy | 73.6 ± 17.3 | 69.7 ± 16.8 | 0.52 |
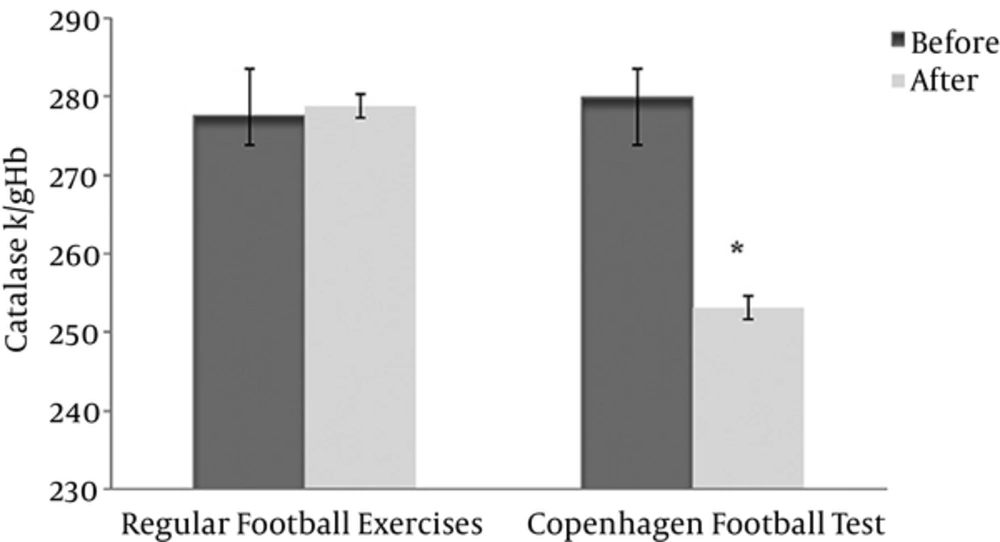
Erythrocyte catalase activity was significantly reduced in the experimental group (before 279.88 ± 26.17 after 253.13 ± 44.34) compared with the control group (before 277.58 ± 35.42 after 278.82 ± 50.34) (P < 0.05, effect size = 0.22) (Figure 1). The results of covariance analysis revealed that according to F observed (intra- group 24.3 and inter-group 19.39) at the level of 0.05, there was a significant difference in the average posttest of catalase activity between the 2 groups after adjusting for the pretest data (P < 0.05).
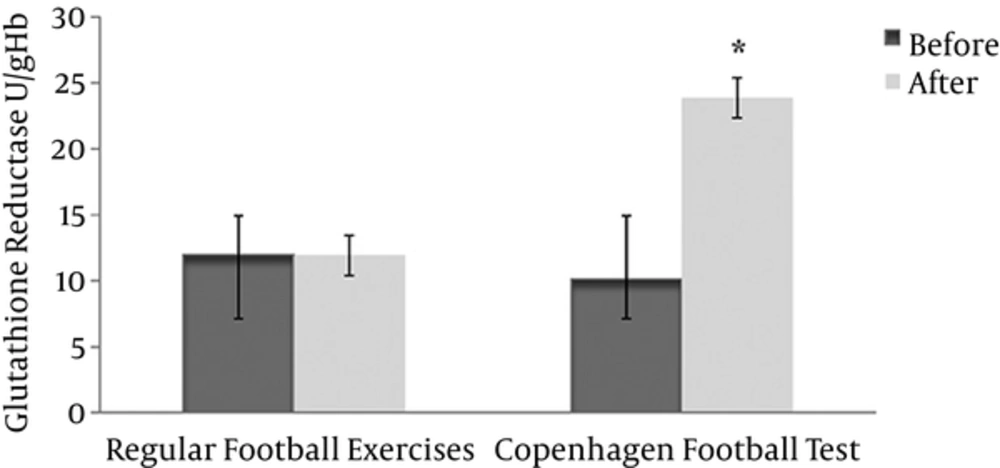
The results of measuring the activity of glutathione reductase in red blood cells showed a significant increase in the experimental group (before 10.13 ± 0.94 after 23.86 ± 5.96) compared with the control group (before 11.96 ± 0.94 after 11.92 ± 1.56) (P < 0.05, effect size = 0.31) (Figure 2). The results of analysis of covariance revealed that according to F observed (intra- group 7.27 and inter-group 0.34) at the level of 0.05, there was a significant difference in the average posttest glutathione reductase between the 2 groups after adjusting for pretest data (P < 0.05).
4. Discussion
In interpreting the results of this study, we should not just pay attention to the superficial differences and similarities of this research with the other researches. Most of the changes in the studies done in this area are due to their methodologies or their participants. It should be noted that Copenhagen-based test have been designed for players in different positions in an attempt to simulate the main conditions of the competition. Therefore, the differences in the running quantity are another factor that must be considered in generalizability of the results.
Ferrer et al. (2009) found that professional players, after adapting to period of training, showed a decrease in the activity of catalase (50%) and glutathione reductase (150% - 200%) in response to competitions (14). It may be possible that the decrease in CAT activity was due to the exercise adaptation as well as vitamin E supplementation that had been reported previously in healthy participants (15). In line with these changes, increased erythrocyte glutathione reductase is essential for the protection of glycolysis (16). However, some studies did not confirm this result (17).
Nonetheless, in this case, the results are not clear and integrated. Perhaps the decrease in CAT activity could be due to environmental use to fight free radicals that are produced in vigorous physical activity. Thus, if this chronic stress is reduced, then it could be considered as a pathological agent (18).
That is why the interpretation of the results, catalase and glutathione reductase activity changes, depends on many factors. Moreover, the methodology of the study and its participants are very important. Even a sudden increase in patients as pathological conditions could fight free radicals and cell damage (19, 20). It seems that in the present study the increase in cellular glutathione reductase stabilized the conditions. Moreover, the decrease in the activity of the catalase enzyme was the result of its fight with free radicals. Eccentric muscle contractions in these changes are very important in Copenhagen test (21).
Many factors could determine whether exercise increases the damaging effects of free radicals, the most important of which is intensity of exercise. Other factors that determine the degree of oxidative stress (free radical damaging effect) are as follow: the preparedness of athletes, fatigue induced by exercise, and athlete’s diet (4). In this study, antioxidative enzyme activity catalase and glutathione reductase showed different results in red blood cells of female football players. Catalase activity was significantly reduced in the experimental group compared to the control group (P < 0.05). Nevertheless, glutathione reductase activity showed a significant increase in the experimental group compared to the control group. Aguilo et al. (2005) found that the reduction of catalase activity in an individual depends on the intensity interval exercise. During their development to meet superoxide, higher cells design and develop advanced protection enzymes (superoxide dismutase, glutathione peroxidase, glutathione reductase and catalase) and non-enzymatic systems (glutathione) to avoid more dangerous radicals (12).
Both catalase and glutathione reductase enzymes play an important role in omitting hydrogen peroxide, but glutathione reductase is preferred because it has been proven that catalase is effective in removing hydrogen reductase when hydrogen reductase production is in excess of glutathione reductase. In most studies, an increase in the activity of the enzyme glutathione reductase was observed after exercise (22). However, some studies have observed an increase in the catalase activity after exercise (23, 24) some observed no change (25, 26), and some found even lower (27) activity that could have implications on the role of the increase in glutathione as coenzyme glutathione oxidase. During the action of glutathione reductase, glutathione is regenerated and converted to oxidized glutathione that turns NADPH to glutathione again by glutathione reductase (2).
Akkus (2011) examined the effects of exhaustive exercise and long-term training on activities of thiobarbituric acid-reactive substances (TBARs), protein carbonyl (PC), total glutathione (GSH), and total superoxide dismutase (SOD) in a research. The results revealed that exercise increases oxygen consumption significantly. Levels of TBARs, PC, and GSH were significantly under the influence of intense training in females and males. Intense training enhances the SOD activity. The interaction was not between time, acute exercise, and group in activity TBARs, PC, GSH, and SOD in males and females (4). The results showed that protein and fat damage do not change in response to acute exercise with aerobic exercise. The exercise program included running at 80% HR max for 60 minutes a day, 5 days a week for 12 weeks. Blood samples were taken at rest and immediately after exhaustive exercise to measure the signs of oxidative stress and antioxidant enzyme activities (SOD, GPX and CAT) in erythrocytes.
Aerobic capacity improved after training through increasing VO2max. The results showed that an exhaustive exercise increases neutrophil superoxide anion production before and after endurance training, but its size was lower after exercise. The significant increase in lipid peroxidation in erythrocyte membranes was observed after exhaustive exercise; however, exercise reduced these effects. SOD and GPX activity increased after resting (28). Catalase activity did not change with exhaustive exercise, while the intense endurance exercise can increase antioxidant enzyme activity and exhaustive exercise reduces the production of neutrophils superoxide (29). Meanwhile, setting antioxidant defense by reducing lipid peroxidation induced by exercise accompanied the erythrocyte membranes. As the intensity of exercise accompanies a higher percentage of maximal oxygen consumption, the amount of glutathione reserves will increase appropriately. The blood glutathione of professional marathon runners is almost 4 times more than that of those who exercise regularly but unprofessionally. The increase in glutathione reductase reserves in the ROS factors is related to H2O2.
Among the antioxidant enzyme systems, 2 antioxidative enzymes of catalase and glutathione reductase are responsible for the omission of H2O2. Comparing H2O2 in catalase and glutathione reductase, it was found that despite the higher combining affinity of glutathione reductase with H2O2, the amount of catalase increases when there is not enough glutathione reductase in cells to collect H2O2. Glutathione reductase plays a role in cytosol of the cell and changes H2O2 to mineral water without producing molecular oxygen. It is likely that H2O2 increases the amount of glutathione during interval training in response to cellular adaptation with this toxic substance compared with the rest period (30).
In general, based on the findings of the present study, it can be concluded that an interval acute exercise session can decrease and increase catalase and glutathione reductase activity in red blood cells, respectively. The increase of glutathione reductase values lead to protection of erythrocyte metabolism. In the current study, increase of glutathione was due to participants' adaptation. Nonetheless, Copenhagen test as a high-pressure activity should be considered because of the reduced activity of catalase in erythrocytes which is associated with the increase in some cardiovascular risk factors.
The results of this study can also be used by researchers and football coaches. In addition to control the football actual situation, future studies should investigate the oxidative stress in those soccer players who use antioxidant vitamin supplements or herbs. In addition, coaches should also note that due to high pressure and maintained tapering, this type of training should be done at least 48 to 36 hours before a major event. Athletes have also used non-enzymatic antioxidant supplements. The findings suggest that the erythrocytes catalase activity response to vigorous physical activity was acute and glutathione response was chronic.