1. Background
Primary dysmenorrhea causes sharp uterine cramps, usually experienced before or throughout menstruation in the lower abdomen, with no specific pelvic pathological conditions (1). Dysmenorrhea is a condition that affects a significant number of women of reproductive age, with prevalence rates ranging from 93% to 45%, but the highest rate is reported in teenagers (1-5). Because dysmenorrhea is considered a natural aspect of the menstrual cycle, women usually tolerate it, it is not reported, and they do not even seek medical care (6, 7). Even according to the available reports, very severe debilitating pain in women for one to three days in each menstrual cycle can lead to being absent in their workplace or school and somehow affect their quality of life and daily work (8, 9). On the other hand, the cause of primary dysmenorrhea has not been fully elucidated, and dysmenorrhea is one of many menstrual disorders that go undiagnosed and untreated. However, an increase in the synthesis of prostaglandins is one of the most accepted causes. Prostaglandins are the most important chemical mediators and play different bodily roles by affecting cell receptors. There are different types of these biologically active molecules, and structurally, they are similar to cholesterol. Also, their secretion is increased in the blood due to inflammatory reactions, infections, injuries, and external factors. One of the possible mechanisms of primary dysmenorrhea is a decrease in progesterone in the final luteal phase stages, resulting in lysosome rupture and subsequently releasing phospholipase A2 from the endometrium, which shows associations with the increased levels of prostaglandins. This increase causes vasoconstriction, contraction of uterine muscles, uterine ischemia, and, ultimately, pain (10-13). Prostaglandin F 2α (PGF2α) and prostaglandin E2 (PGE2) cause dysmenorrhea, and this increase in prostaglandins has been observed in the menstrual blood of women with dysmenorrhea (14). Another factor affecting dysmenorrhea is weight gain, especially fat tissue in the central areas of the body. Obesity disrupts the balance of steroid hormones, such as androgens, estrogen, and globin, that bind to sex hormones. Consequently, it can cause an increase in estrogen production, which is associated with body weight and fat content. In addition, gastrointestinal symptoms, such as nausea, vomiting, and diarrhea, have been reported in many women with dysmenorrhea caused by PGF2α and PGE2 (15-17). Common treatment methods, including prostaglandin inhibitor drugs, birth control pills, calcium channel blockers, electrical stimulation through the skin, medicinal plants, and massage, are usually used to improve dysmenorrhea. Although their outcomes are still not properly proven, most of these methods are expensive, time-consuming, and sometimes associated with side effects (13, 18, 19). As more focus is being put on women’s health, researchers are looking into cheaper and more effective ways to treat women’s health problems. In this regard, regular physical activity has been reported as one of the useful ways without side effects to prevent and reduce pain caused by dysmenorrhea. Based on the evidence, decreased dysmenorrhea symptoms in women who had regular physical activity are probably due to the effect of hormonal changes on the lining of the uterus or an increase in the level of endorphins. In other words, exercise may act as a non-specific pain reliever (12, 20-22). Despite this evidence, a large body of research supports the positive effects of yoga (especially poses, breathing exercises, and deep relaxation) that can help reduce dysmenorrhea (23-25). In this regard, Kirca and Celik reported that yoga exercises can help reduce menstrual pain in women with primary dysmenorrhea (26). However, disagreements about the effectiveness of exercise activities and yoga training on dysmenorrhea due to using different exercise programs with different quality, intensity, and duration have led to contradictory results in this field (27-29). Although some studies indicate that yoga may alleviate dysmenorrhea, more studies with strong scientific methods are needed to ensure that yoga is a safe and reliable treatment for menstrual health issues such as dysmenorrhea. Because chemical medications can have negative effects and are not recommended in certain situations, more people opt for alternative treatments, specifically minerals known to improve women’s health and overall well-being (30). Some studies showed that zinc might treat menstrual pain and alleviate primary dysmenorrhea (31, 32).
In this regard, Kashefi et al. assessed healthy teenage girls who received 220 mg zinc sulfate capsules for four days, starting the day before the menstruation onset until the third day of bleeding, and reported an improvement in the primary dysmenorrhea (33). The main mechanism by which zinc supplementation may contribute to the relief of menstrual pain is still not precisely understood. However, previous evidence suggests that zinc may have antioxidant and anti-inflammatory effects on the uterus because it can reduce the production of prostaglandins and generally relieve menstrual pain. More scientific studies are needed to determine the potential role of zinc supplements in reducing primary dysmenorrhea pain, and it is still premature to draw accurate conclusions (31, 34).
2. Objectives
Considering the importance of primary dysmenorrhea, focusing on pain relief interventions with minimal side effects, such as yoga exercises, and consuming a vital element such as zinc to improve the body’s reactions is crucial, particularly because its serum levels are reduced in primary dysmenorrhea. Moreover, the effectiveness of these two interventions has been investigated separately in previous research. This study aimed to investigate the effects of yoga exercises and zinc sulfate supplementation on the level of prostaglandin E in non-athletic girls with primary dysmenorrhea over three ten-day courses.
3. Methods
3.1. Subjects and Study Procedure
The present quasi-experimental research had a pre-test and post-test design. After an advertisement was published in the Fardis district Karaj healthcare centers, the subjects who referred to these centers expressed interest in participating in the current research. Seventy young girls volunteered to participate in the present study (Age: 18 - 25, BMI: 21 - 25 kg/m2, Weight: 54 - 65 kg). Entry criteria comprised having primary dysmenorrhea based on the diagnosis and opinion of a gynecologist, being single, aged 18 to 26, and not having a history of regular physical activity and exercise during the previous years. In addition, other criteria such as no smoking history, hypotension or hypertension, consuming anabolic steroids or drugs stimulating the sympathetic nervous system (SNS) and the central part of the adrenal gland or specific drugs, low-calorie diets, and knee injury were considered. Exclusion criteria included having a history of second dysmenorrhea based on the diagnosis of a gynecologist, a history of diseases related to restrictions on participation in exercise, pregnancy, known diseases related to the reproductive system, suffering from chronic diseases (cardiovascular disorders, respiratory problems, chronic kidney abnormalities, type 2 diabetes mellitus, thyroid disorder, anemia symptoms, neurological and mental disorders), participation in other sports fields, dissatisfaction with continuing to participate in research, failure to complete research questionnaires, the occurrence of unpleasant problems and stressful events during the research process and non-participation in three consecutive sessions or four alternating sessions of yoga exercises during the study. Eventually, all participants completed and signed the questionnaire, medical history, and consent forms. The subjects received explanations of the overall process of the test and the measurement methods to become familiarized with the research process. Also, they were given explanations about not using ergogenic aids, such as creatine monohydrate, protein, supplements, and different drugs during the research process. They were also expected to refrain from any strenuous activity for at least 72 hours before the experimental sessions, all of which considered exclusion criteria.
3.2. Ethical Considerations and Sampling Technique
All used protocols in the current research were implemented according to the international ethical guidelines for biomedical research on human subjects developed by the International Council of Organizations of Medical Sciences (CIOMS) with the cooperation of the World Health Organization (WHO; Geneva, 2016) [35]. The Islamic Azad University of Karaj Ethics Committee approved the study (IR.IAU.K.REC.1398.078). The sample size was considered based on an earlier investigation using G*Power software version 3.1 n = 70, calculated using α = 0.05 and β = 0.2 (20). Even though the researchers had strict and complete supervision on how to conduct the current research process, in the end, 22 people due to reasons such as simultaneous use of other drugs (5 people), taking some sports supplements irregularly (4 people), not attending yoga exercises regularly (3 people), and 3 people from the group were excluded. As for the control, due to not completing the questionnaires, 3 people were excluded from the present study due to unwillingness to continue participating in the research, 2 people due to injuries, and 1 person was excluded due to other reasons. Finally, 48 people participated in the research process and completed data extraction.
3.3. Procedures for Randomization
The people taking part in the study were split into four groups of 12 subjects (placebo, yoga and placebo, zinc sulfate, and yoga and zinc sulfate) randomly using a lottery. Someone who wasn’t part of the study put individual numbers in small plastic unclear capsules. These capsules were then put into a vase. The people taking part in the study and the investigators conducting it did not know which group they were assigned to until the lottery began and the capsules opened. The allocation of groups was kept a secret. Ultimately, the main researcher wrote down the names of the participants in each group after choosing them randomly. Moreover, they again explained the study’s important details to each group (35).
3.4. Anthropometric Measurements
The subjects’ height was determined by the Seca brand medical height meter, produced in Germany with a 0.01-meter assessment accuracy. The participants were not wearing shoes and had to stand, so their heels, hips, shoulders, and heads touched a rod for calculation. They also had to ensure that their heads were in a straight line with the horizon. Afterward, they measured the subjects’ height by placing a stadiometer on top of their head and looking at the measurement. We only took readings between eight and nine in the morning to ensure correct weight measures. The subjects’ weight was measured using a German scale called Soehnle, which is used for medical purposes. This scale is precise and can measure weight with an accuracy of 0.1 kg. To measure the participants’ weight, they were asked to wear as little clothing as possible (T-shirts and sports pants, but not shoes) and then weighed. We calculated the body mass index (BMI) by dividing participants’ weight in kilograms by their height in meters squared.
3.5. Dose and Blinding of Zinc Sulfate Supplements
During the study, the people in the supplement groups were given capsules containing zinc sulfate daily after exercise at 10:30 a.m. (in three consecutive menstrual periods, from the 22nd day of menstruation to the third day of bleeding (for ten days)). The capsules contained 220 mg of zinc sulfate (Ramopharmin Co, Tehran, Iran) (33, 36). In contrast, the group that received the placebo daily consumed empty capsules that looked the same and contained lactose. Someone not part of the study prepared the bottles for the participants. They put zinc sulfate and placebo into the bottles. The bottles looked the same, and each had a number to identify which participant it belonged to. These bottles were then given to the researchers. However, this person did not give the capsules to the people involved in the study. Both people and investigators who handed out the capsules and collected data needed to know what was in each bottle. Additionally, the analysis was performed in the laboratory without knowing which bottles had which substances.
3.6. Technique and Time for Blood Sampling
We took blood samples from all four groups to check the specific biochemical levels. We did this twice, once before they started yoga exercises and once again after they had completed three sets of ten-day yoga courses. They needed to fast for 12 to 14 hours before giving the blood samples. At the beginning of the blood collection, everyone was told not to do intense exercise, avoid stressful situations, and not take any drugs for three days before the test. When the participants arrived at the lab between 8:00 and 10:00 a.m., they sat for five minutes. Then, the lab technician used a 10-cc syringe from Saha Company to collect 7 mL of blood from the median cubital vein of the right hand. We divided the blood sample into two parts: Serum and plasma. Serum was obtained by letting the sample coagulate and using a Hettich centrifuge from Germany that spins at 3000 revolutions per minute for 15 minutes. Plasma was prepared by adding an anticoagulant called (Ethylenediamine tetra-acetic acid) EDTA from Merck (Germany), so the selected variables were measured. For additional testing, some serum samples were kept in an American-made 6UX ULT freezer at - 80 degrees Celsius. After finishing this step, the subjects experienced the independent factors. After that, the program ended, and all participants were asked to come to the lab again 48 hours after their last training session. This was done to ensure that any immediate changes in their blood were not due to the training session. They were also asked to provide a blood sample like they did initially. Following blood collection and separation, appropriate laboratory equipment and validated laboratory procedures were used to measure pertinent data for each studied parameter and marker. In this regard. In the pre-test and post-test, the serum levels of prostaglandin E were measured by the ELISA method according to the kit protocol (Novus Biologicals, Centennial, CO, USA). Also, blood samples were analyzed by the Cobas Integra analyzer (Roche Diagnostics, Germany), and the levels of prostaglandin E were determined.
3.7. Exercise Training Program (Yoga)
The yoga group performed yoga exercises for 60 minutes a day (9:00 - 10:00 a.m.) in three consecutive menstrual periods (12 weeks), from the 22nd day of menstruation to the third day of bleeding (for ten days). A qualified yoga instructor was in charge of all the yoga classes and taught the yoga poses clearly and consistently. Participants who were unable to perform the standard postures were taught modified poses. It was emphasized that the exercises should be done in a calm, quiet environment, with an appropriate temperature and suitable flooring, while wearing comfortable, loose clothing in the morning, if possible, with an empty bladder and bowel. The yoga exercise program started with a 10-minute warm-up. Then, there were 20-minutes of doing postures called asanas. After that, there were 20-minutes of respiratory exercises called pranayama. Next, there was a 5-minute relaxation period. Finally, there was a 5-minute meditation time. The sequence and duration of the yoga techniques practiced by the subjects can be seen in Table 1.
| Structure | Components of Exercise | Time in Minutes |
|---|---|---|
| Warm‑ups | Five minutes of walking at a medium speed and deep inhalation and exhalation and ends with five minutes of static stretching exercises | 10 |
| Asanas | Doing postures | 20 |
| Marjariasana | Cat stretch pose | 4 |
| Ardha Pawanmuktasana | Half wind release pose | 4 |
| Balasana | Child’s pose | 4 |
| Paschimottanasana | Seated forward bend pose | 4 |
| Shavásana | Corpse pose | 4 |
| Pranayama | ||
| Brahmari Pranayama | The ability to take deep breaths and exhale deeply while humming was instructed to the subjects. | 5 |
| Sheethali Pranayama | Subjects were instructed to draw air slowly and deeply through a curled tongue extended out of the mouth. They were instructed to passively exhale through the nose after the inspiration when the tongue was pulled back and the mouth was shut. | 5 |
| Relaxation | The participants were told to stay still in a lying down position known as the corpse pose. | 5 |
| Meditation | The participants should pay close attention to how their bodies move. | 5 |
| Total time | 60 |
Session of Yoga Exercises by the Subjects During the Training Schedule
3.8. Statistical Analysis
The obtained data were analyzed using descriptive and inferential statistics methods. Mean and standard deviation were used for the descriptive statistics. Regarding inferential statistics, after examining the normal distribution of data using the Shapiro-Wilk test, the hypotheses were tested using a two-way analysis of covariance. Data analysis was done using IBM SPSS 23 at the significance level of P < 0.05.
4. Results
4.1. Anthropometrics Measurements
Some anthropometric characteristics and the comparison of these characteristics are shown in Table 2 (after ensuring the data normality using the Shapiro-Wilk test, comparisons were made using one-way ANOVA).
Based on the results obtained in Table 2, the four investigated groups had no significant differences in terms of the investigated variables of age, height, weight, and BMI (P > 0.05). This showed that assimilation was done in terms of the variables examined in Table 2 in the present four groups.
| Variables | Groups | Sig | |||
|---|---|---|---|---|---|
| Placebo | Zinc Sulfate | Yoga and Placebo | Yoga and Zinc Sulfate | ||
| Age (y) | 20 ± 1.54 | 21.42 ± 1.83 | 22 ± 2.59 | 21.33 ±2.43 | 0.15 |
| Height (cm) | 163.25 ± 6.58 | 163.25 ± 4.35 | 162.5 ± 6.22 | 163.83 ± 4.78 | 0.95 |
| Weight (kg) | 56.25 ± 5.58 | 59.08 ± 5.09 | 55.5 ± 7.23 | 54.25 ± 7.56 | 0.32 |
| BMI (kg/m2) | 21.18 ± 2.42 | 22.23 ± 2.46 | 21.11 ± 3.28 | 20.15 ± 2.16 | 0.3 |
Comparison of Some Anthropometric Characteristics of Subjects of the Four Groups in the Pre-test Stage
4.2. Prostaglandin E
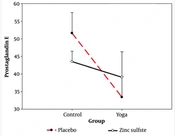
Figure 1 shows different groups’ mean and standard deviation regarding the variable prostaglandin E.
As shown in Figure 1, in the pre-test stage, the highest and lowest averages of prostaglandin E belonged to the placebo (50.25) and zinc sulfate (47.08) groups, respectively. In the post-test stage, the highest and lowest averages of prostaglandin E were related to the placebo (51.58) and yoga (33.42) groups, respectively.
Since a pre-test was taken in this study before the interventions, to control the effects of covariance (pre-test), analysis of covariance was used. Also, because two independent variables, including exercise with two levels (yoga exercise and no exercise) and supplement with two levels (zinc sulfate supplement and placebo), were applied, a 2x2 design was used. Therefore, two-way covariance analysis was utilized for hypothesis testing. Table 3 shows the results obtained from this test.
| Variables | Sum of Squares | df | Means of Squares | F | Sig | Eta |
|---|---|---|---|---|---|---|
| Zinc sulfate | 8.175 | 1 | 8.175 | 0.278 | 0.601 | 0.006 |
| Yoga | 1502.834 | 1 | 1502.834 | 51.057 | 0.001 | 0.543 |
| Zinc sulfate and yoga | 516.995 | 1 | 516.995 | 17.564 | 0.001 | 0.290 |
| Error | 1265.670 | 43 | 29.434 |
Analysis of Covariance Test Results Related to Prostaglandin E
Table 4 shows the main effect of supplementation and without the main effect of exercise.
In this test, the main effect of the supplement did not show significance (F1, 43 = 0.278, P = 0.601, η = 0.006). Therefore, zinc sulfate supplementation could not change serum prostaglandin E levels.
Table 5 shows the main effect of yoga exercise without the supplement’s main effect.
This test showed the significance of the main effect of yoga (F1, 43 = 51.07, P = 0.001, η = 0.543). Yoga exercise reduced serum prostaglandin E levels in three to ten days.
Table 6 shows the average interactive effect of yoga exercise and zinc sulfate supplementation on the serum prostaglandin E variable.
Mean and Standard Deviation of the Interactive Effect of Yoga Exercise and Zinc Sulfate Supplementation
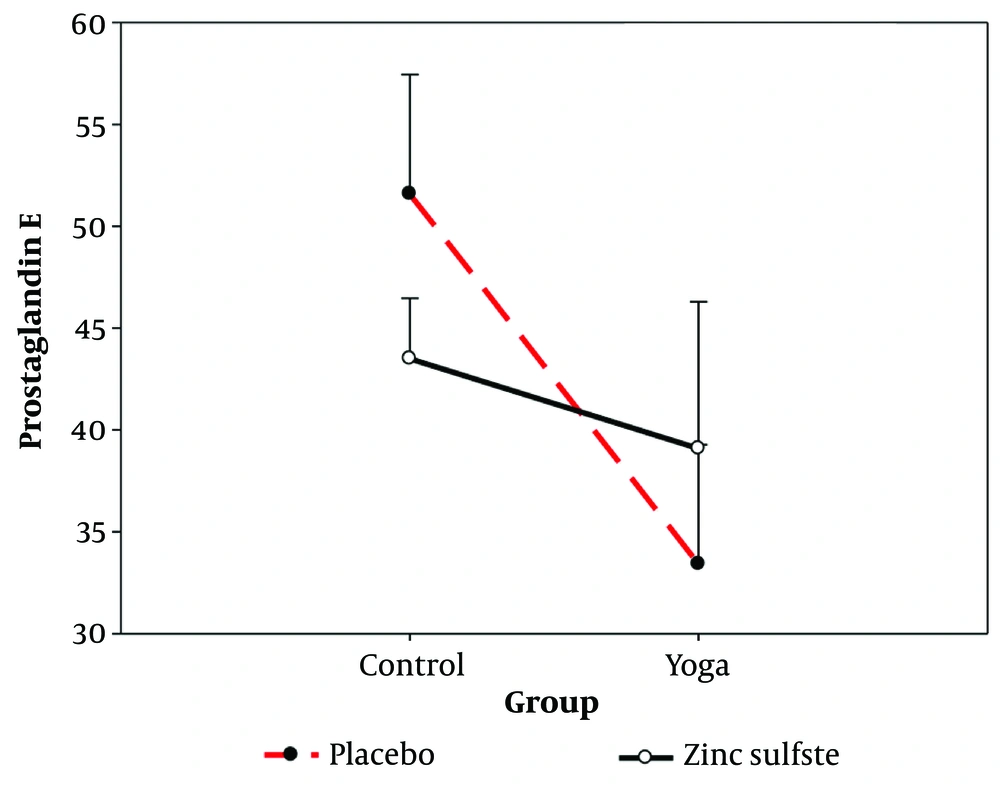
The results of two-way covariance analysis showed that there was an interaction effect between yoga practice and zinc sulfate supplementation on the serum level of prostaglandin E in non-athlete participants (F1, 43 = 17.567, P = 0.001, η = 0.290) (Figure 2).
As shown in Figure 2, the average of the exercise groups (yoga) was lower than those that did not exercise. When the subjects did not exercise, they had less prostaglandin E if they consumed zinc sulfate than the placebo group. In contrast, with yoga exercise, the result was different, and the use of placebo reduced prostaglandin E more than the zinc sulfate supplement. According to the results, the main null hypothesis that yoga exercises and zinc sulfate supplementation do not significantly affect prostaglandin E in non-athlete women was rejected, and the statistical hypothesis was accepted.
5. Discussion
The current study investigated the effect of three ten-day yoga exercise courses and zinc sulfate supplementation on prostaglandin E in non-athletic women with primary dysmenorrhea. The study’s critical purpose was to determine whether this combination could significantly decrease the prostaglandin E levels.
Even though dysmenorrhea’s pathophysiology is poorly understood, a rise in uterine prostaglandin production is thought to be one of the main causes of primary dysmenorrhea. The prostaglandins increase vasoconstriction and myometrial contractions, which causes uterine ischemia and the synthesis of anaerobic metabolites. This makes pain nerves more sensitive, leading to pelvic pain (6, 37, 38). In this regard, evidence in the present study demonstrated that in the pre-test stage, the highest averages of prostaglandin E belonged to the placebo group. In line with our results, Jeong et al. have reported increased prostaglandin levels in dysmenorrhea and heavy menstrual bleeding. Based on this study, it has been demonstrated that severe dysmenorrhea and menstrual flow are strongly correlated, which was most likely because prostaglandin levels were rising (39). Even in previous studies, it has been stated that the cause of primary dysmenorrhea is mainly linked to the role of prostaglandins and leukotrienes. In general, the blood flow in this area is reduced or stopped along with the contraction of the uterus by prostaglandins. Along with this process, the amount of blood perfused to the uterus by myometrial compression of blood vessels drops. In the end, the uterus does not receive enough oxygen, leading to cramping and pain in the abdominal area (31).
Also, in the current study, evidence showed that when subjects did not exercise, if they only consumed zinc sulfate, they had lower prostaglandin E levels than the group that only consumed placebo. While we removed the main effect of supplementation, we observed that yoga exercise was also able to reduce serum prostaglandin E levels in three ten-day periods. On the other hand, we found an interaction effect between yoga practice and zinc sulfate supplementation on decreasing serum levels of prostaglandin E in non-athletes. Previous studies have pointed out the importance of zinc supplementation and yoga exercise in modulating prostaglandin levels separately. In agreement with this study, Obiagwu et al. conducted research on students aged between 13 and 21 with primary dysmenorrhea and observed that the consumption of 40 mg of zinc sulfate during three intake periods diminished the incidence and intensity of primary dysmenorrhea (30). In addition, the results of the research conducted by Eby showed that the consumption of zinc improves dysmenorrhea and cramps in the uterus, which is in line with the evidence obtained in the present study (34). In another study, Goei and colleagues, in 1982, during an investigation, found that when patients took 31 mg of zinc every day, they did not experience premenstrual tension symptoms. However, when patients only took 15 mg of zinc, they did experience these symptoms (40). Additionally, a study was conducted by Chuong and Dawson to determine whether variations in zinc levels are related to indications of premenstrual disorder symptoms (PMS). Compared to controls, patients with premenstrual disorder appeared to have lower zinc concentrations amid the luteal stage than they did amid other stages of the cycle, indicating the possible role of zinc deficiency (41).
Several theories may justify these beneficial effects on improving dysmenorrhea. Zinc can reduce the production of prostaglandins and inhibit and suppress the metabolisms dependent on prostaglandins and leukotrienes, which are known causes of dysmenorrhea. It can ultimately reduce uterine cramps (34, 42-44). Also, the reduction of cyclooxygenase 2 (COX-2) activity by zinc supplementation has been shown in in vivo investigations (45, 46). Based on the previous evidence, supplementing with zinc appears to help by regulating an enzyme called cyclooxygenase-2 (COX-2), which is involved in pain, inflammation, and some cancers, such as uterine cancer. It can also reduce dysmenorrhea-related complications (34, 36, 47). Zinc also appears to have antioxidant and anti-inflammatory properties because, according to the available evidence, its consumption can moderate the inflammation caused by dysmenorrhea and increase blood flow in the uterus arteries (40, 48, 49).
On the other hand, due to the positive effects of physical activity, it can be relatively effective on dysmenorrhea. Regular physical activity can help reduce stress, anger, depression, discomfort, and total severity of PMS and possibly improve blood flow in the pelvis. Continuous exercise increases blood endorphin levels, reduces adrenal cortisol, and provides pain relief for short periods. Also, evidence indicates that regular yoga exercises improve conscious control over autonomic functions of the body so that even these exercises eliminate muscle and nervous tension and lead to an increase in energy levels. Therefore, the yoga exercises used in the present study caused the same effect and could address the painful conditions in women with primary dysmenorrhea (20, 50-52). The results obtained by the present study agree with some evidence obtained by previous researchers. In this regard, Kamalifard et al. reported that doing yoga for ten weeks in three 60-minute sessions could reduce the symptoms of premenstrual syndrome in women and alleviate pain (53). Similar results were reported by Rakhshaee, indicating a significant reduction in the primary dysmenorrhea pain intensity and duration in the yoga group compared with the control (54). The women between 18 and 25 who participated in the study by Yang and Kim completed a 60-minute yoga program once a week for 12 weeks, including physical exercises combined with relaxation and meditation. They reported reduced menstrual pain and discomforts caused by primary dysmenorrhea (24). Concerning the investigation of Yonglitthipagon et al., women with primary dysmenorrhea aged between 18 and 22 who practiced yoga (12 weeks/twice a week/30 min per day) at home were able to control the severity of menstrual pains and improve physical wellness and the quality of life. Therefore, they proposed that yoga may be a conceivable complementary treatment for primary dysmenorrhea (25). Concerning the role of yoga exercise on dysmenorrhea, the evidence shows that it can suppress pain, along with the reduction of prostaglandin production and inhibition of myometrial ischemia. Based on these results, adjusting the stress pathways through yoga exercises can maintain the hormonal balance and improve dysmenorrhea (12, 55). Altogether, for women with primary dysmenorrhea, exercise yoga is an effective treatment for reducing menstrual pain (28).
The present study had several limitations, such as failure to measure inflammatory factors, beta-endorphin hormones, cortisol, and other enzymes involved in the mechanism of primary dysmenorrhea. Other limitations of this investigation include a small sample size and the perception of menstrual pain recorded by a visual analog scale (VAS). This should be considered in future research. According to the evidence mentioned in previous studies and the evaluation of prostaglandin E levels in the present study, regular participation in yoga exercises and consuming zinc supplementation can be a suitable and low-cost strategy to reduce and improve primary dysmenorrhea complications in young women. In addition, other limitations were short study duration, lack of follow-up, and measurement of a single prostaglandin. Future studies should consider these limitations.
5.1. Conclusions
Based on the present research results, three ten-day yoga exercise courses combined with zinc sulfate supplementation could modulate prostaglandin E and may help alleviate primary dysmenorrhea complications. However, more accurate and definitive results seem to need more research on different doses of zinc supplementation along with different methods of yoga exercises in a larger cohort.