1. Background
Osteoporosis is a common systemic skeletal disorder (1), which has devastating effects on skeletal structure and general health and can even cause the death of the affected person; it is, therefore, considered the fourth threatening disease of humans, after heart attack, stroke, and cancer (2, 3).
According to a systematic review and meta-analysis in 2020, more than 18% of the world’s population suffer from osteoporosis or the high risks of its related fractures (especially in the femur and spine) (4), and its prevalence is consistently rising (a 23% increase from 2010 to 2025) (5).
There are some modifiable (nutrition, physical activity, etc.) and non-modifiable (age, sex, etc.) factors in osteoporosis prevention (4). Sports activity is one of the best modifiable factors in improving bone mineral density (BMD) (6, 7). In general, sports can be divided into 2 categories: Weight-bearing (6) and non-weight-bearing (7). Although the first category is more effective (6), weight-bearing sports are not suitable for everyone since an athlete with osteoporosis, apart from the risk of fractures, may also have cardiovascular and cerebrovascular diseases (8).
Due to the many therapeutic effects of swimming (a non-weight-bearing sport), such as improving the cardiopulmonary and metabolic systems, it is considered the best option for most people (7). However, there is still conflicting evidence of its effects on bone health. Some researchers found it to be a helpful sport (7, 9), while others reported it as an ineffective or neutral activity (10). Therefore, swimmers are always worried about the development of osteoporosis (5).
Unfortunately, most previous studies did not separate the results of males and females, which can be a potential explanation for their contradictory outcomes (11, 12). As mentioned before, sex difference is one of the main non-modifiable elements affecting the changes in bone status and life expectancy of osteoblasts/osteoclasts (13, 14).
Unlike the models for rodents that reported a greater improvement in BMD of males than females (15, 16), human information about the uniqueness of mechanical loadings’ effects caused by a swimming practice on the skeletal systems of males and females is not clear (11, 12, 17). It is obvious that elite swimmers have to spend many hours practicing in their adolescence and youth. Thus, it will clear any damaging or productive changes of bone tissue in a skeletal framework caused by hypo-gravity conditions (5).
Limited studies have compared the bone variables of elite swimmers with non-athletes (NA) (5, 18), and very few surveys focused on the effect of swimming according to swimmers’ sex (11, 12, 17). Besides, as far as we know, there is no study to compare the bone characteristics (BMD, T-score) of elite male swimmers with their female counterparts (18 to 24 years). Therefore, the present study was conducted to reach the mentioned aims.
2. Objectives
The aims of this study were to compare the bone mineral density (BMD; lumbar spine and proximal femur areas) of elite swimmers with NA and compare the corresponding values in male (MS) and female swimmers (FS). It is assumed that there is no significant difference between the BMD of swimmers and NA (10); also, MS have better bone acquisition than FS (12).
3. Methods
3.1. Study Design
The cross-sectional study compared the effect of swimming on the BMD values of elite MS with FS and male and female NA. The study was conducted according to the Declaration of Helsinki 2018 and approved by the Ethics Committee of the Sport Sciences Research Institute of Tehran, Iran (19).
3.2. Participants
In total, 56 individuals participated in the present study, 28 of whom were elite MS and FS who were members of the Iranian national swimming team, and 28 were male and female NA. They all enrolled voluntarily and were purposively divided into 4 groups according to their activities: MS (n = 14), FS (n = 14), male NA (n = 14), and female NA (n = 14). G*Power v. 3.1 software (power of 80%) was used to calculate the sample size, with a significance level of 0.05. A total of 44 participants were needed for the study.
3.3. The Inclusion and Exclusion Criteria
Inclusion criteria: All swimmers (speed and endurance) in the age range of 18 to 24 years old, with more than 8 years of training in swimming, practice time of at least 11 - 14 hours per week (6 days/week, 2 - 3 hours/day, 11 months/year), and with experience of participating in notable teams and attending national or international matches for at least 3 years. The participants were monitored by the nutrition department to ensure a balanced diet and intake of all basic nutrients during their training season, and they were also re-evaluated monthly to control their nutritional intake (5). Moreover, the first menstruation of all the female participants was before the age of 14 years (11). All the NA had their normal activities of daily living; they had no regular special physical practices during or before the study.
Exclusion criteria: The subjects were excluded if they had one of the following factors: Bone diseases, hypo- or hyperthyroidism, parathyroid disease, kidney failure, respiratory heart disease, diabetes, liver failure, smoking, drinking alcohol (5), taking drugs affecting BMD (e.g., testosterone, corticosteroids), previous participation in a weight-bearing sport (e.g., soccer, volleyball,), using any kind of contraceptive drug for delaying the menstrual period (in female subjects) for more than 3 months, and history of hormonal (e.g., thyroid and testosterone) and nutritional (e.g., obesity and eating) disorders which affect BMD (4).
3.4. Data Collection Tools
A meter and an analog weighing scale (a sensitivity of 0.1 kg) were used to measure the subjects’ height and weight, respectively. Then, the body mass index (BMI) was calculated by using the formula: (Weight (kg)/height (m2)). The DEXA scan machine (Hologic Series Discovery QDR, Software Physician’s Viewer, APEX System Software v. 3.1.2. Bedford, MA, USA) was employed as a gold standard method to measure the BMD values (g/cm2) of the lumbar spine (L2 - L4) and proximal femur (neck, trochanter, and Ward’s triangle) areas (14). The participants’ T scores were also calculated (20). According to the World Health Organization (WHO), T-scores provide reference information about the same sex and young people in the population. If the T-scores are between - 1 and - 2.5 standard deviations (SDs), the individual suffers from osteopenia, but if these scores are less than - 2.5 SDs, the person is considered to have osteoporosis (21). All the subjects were well informed about the procedure and possible side effects of the scan before performing the test. Two experienced and skilled technicians performed the scan with the DEXA machine and analyzed its data on different bone areas.
3.5. Statistical Analysis
The statistical analysis was performed by using SPSS v. 21.0 (SPSS Inc., Chicago, IL). All the data in the tables and the text are presented as mean ± SD. The data were analyzed by using the analysis of covariance (ANCOVA). Bonferroni’s post-hoc test was also used to compare the means of the variables, and the significance level was set to P ≤ 0.05.
4. Results
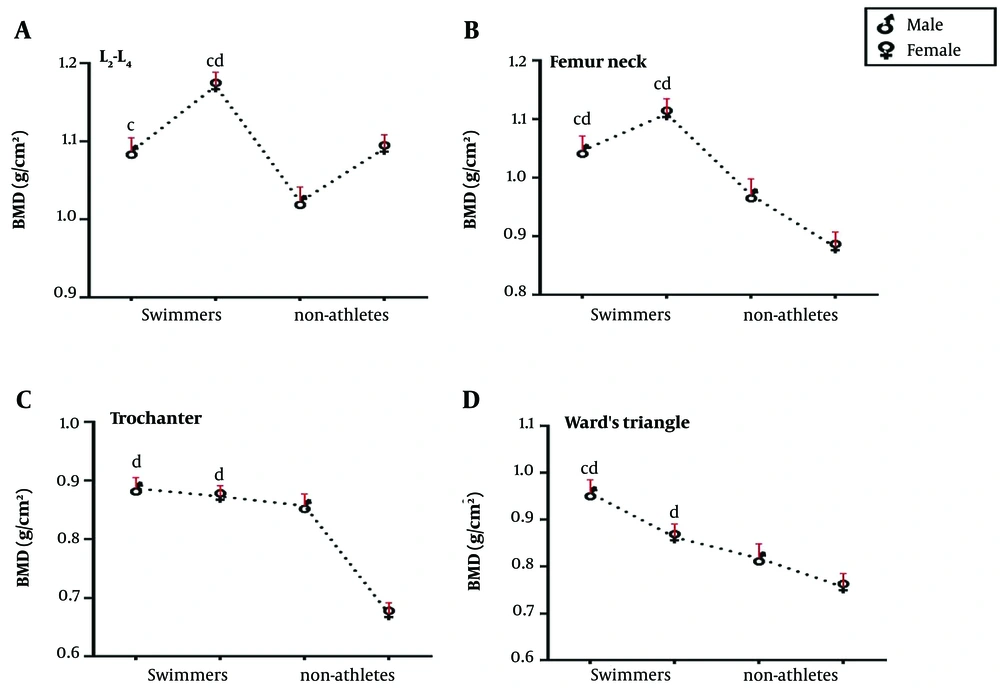
The demographic data (e.g., age and height) were compared between groups by using the analysis of variance (ANOVA). There was a significant difference between the demographic factors of male subjects and those of the female ones, as the males were taller and heavier, with lower body fat percent and higher BMI than females (all P < 0.001). The data distribution homogeneity was confirmed using the Shapiro-Wilk test. Then, a one-way ANCOVA was applied to compare the BMD and T-Score in the 4 groups. The results showed a significant difference in the BMD means of L2-L4 (F(3, 38) = 13.91, P < 0.001, η2 = 0.52), femur neck (F(3, 38) = 36.01, P < 0.001, η2 = 0.74), trochanter (F(3, 38) = 55.02, P < 0.001, η2 = 0.81) and Ward’s triangle (F((3, 38) = 66.47, P < 0.001, η2 = 0.52), and the T-score means of L2-L4 (F(3, 38) = 21.63, P < 0.001, η2 = 0.63) and femur neck (F(3, 38) = 91.57, P < 0.001, η2 = 0.88) of the 4 groups (Table 1).
| Demographics | (N = 14) | P-Value | |||
|---|---|---|---|---|---|
| MS | FS | MNA | FNA | ||
| Age, y | 22.83 (1.34) | 19.92 (0.90) | 24.40 (0.97) | 23.10 (1.20) | < 0.001 |
| Height, cm | 184.5 (5.28) | 166.8 (0.75) | 187.0 (4.94) | 167.6 (2.79) | < 0.001 |
| Weight, kg | 83.17 (8.36) | 55.75 (0.97) | 80.60 (8.87) | 55.50 (2.68) | < 0.001 |
| Body mass index, kg/m2 | 24.36 (1.10) | 20.05 (0.36) | 23.02 (2.13) | 19.76 (0.40) | < 0.001 |
| Body fat, % | 18.28 (1.40) | 23.24 (0.61) | 17.04 (2.45) | 23.62 (0.39) | < 0.001 |
| Test variables (unadjusted) | |||||
| BMD L2-L4, g/cm2 | 1.16 (0.07) | 1.10 (0.01) | 1.10 (0.00) | 1.02 (0.05) | < 0.001 |
| BMD femur neck, g/cm2 | 1.11 (0.09) | 1.07 (0.03) | 1.01 (0.07) | 0.83 (0.02) | < 0.001 |
| BMD trochanter, g/cm2 | 0.89 (0.01) | 0.89 (0.07) | 0.82 (0.04) | 0.68 (0.01) | < 0.001 |
| BMD Ward’s triangle, g/cm2 | 0.98 (0.04) | 0.86 (0.09) | 0.80 (0.05) | 0.75 (0.03) | < 0.001 |
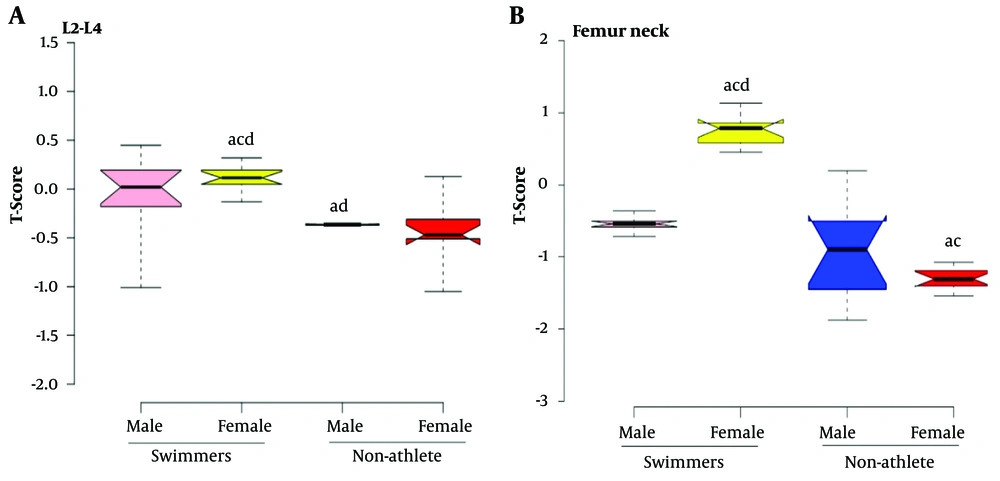
| T-score L2-L4 | -0.04 (0.39) | 0.10 (0.13) | -0.36 (0.01) | -0.46 (0.32) | < 0.001 |
| T-score femur neck | -0.54 (0.09) | 0.76 (0.20) | -0.89 (0.71) | -1.30 (0.13) | < 0.001 |
| Test variables (adjusted) | |||||
| BMD L2-L4, g/cm2 | 1.09 (0.02) | 1.17 (0.02) | 1.02 (0.02) | 1.09 (0.02) | < 0.001 |
| BMD femur neck, g/cm2 | 1.05 (0.03) | 1.11 (0.03) | 0.97 (0.03) | 0.88 (0.03) | < 0.001 |
| BMD trochanter, g/cm2 | 0.89 (0.02) | 0.87 (0.02) | 0.86 (0.02) | 0.67 (0.02) | < 0.001 |
| BMD Ward’s triangle, g/cm2 | 0.96 (0.03) | 0.87 (0.03) | 0.82 (0.03) | 0.76 (0.03) | < 0.001 |
| T-score L2-L4 | -0.43 (0.10) | 0.51 (0.11) | -0.78 (0.11) | -0.69 (0.11) | < 0.001 |
| T-score femur neck | -0.55 (0.13) | 0.53 (0.14) | -0.50 (0.15) | -1.42 (0.14) | < 0.001 |
The Comparison of Demographic Characteristics, Adjusted and Unadjusted BMD, and T-Score a
The results showed a significant difference in the mean of L2- L4 BMD (F(3, 38) = 13.91, P < 0.001, η2 = 0.52), femur neck BMD (F(3, 38) = 36.01, P < 0.001, η2 = 0.74), trochanter BMD (F(3, 38) = 55.02, P < 0.001, η2 = 0.81), Ward’s triangle BMD (F(4, 50) = 66.47, P < 0.001, η2 = 0.52), L2- L4 T-score (F(3, 38) = 21.63, P < 0.001, η2 = 0.63) and femur neck T-score (F(3, 38) = 91.57, P < 0.001, η2 = 0.88) in the 4 study groups.
The results of the post-hoc test are shown in Figure 1. The L2-L4 BMD in MS was significantly higher than in male NA (P < 0.01), and the same variable was significantly higher in FS than in NA (P < 0.001). While the BMD of swimmers in the femur neck was significantly higher than NA (P < 0.05), the trochanter BMD was significantly higher in swimmers than female NA (P < 0.001). Ward’s triangle BMD of swimmers was significantly higher than female NA (P < 0.01), but just Ward’s triangle BMD of MS was significantly higher than male NA (P < 0.001). Figure 2 presents the results of the post-hoc test for comparing the means of T-scores in 4 groups. It is clear that L2-L4 and femur neck T-scores in FS were significantly higher than in MS and NA groups (P < 0.001), and the L2-L4 and femur neck T-score in male NA was significantly lower than in MS (P < 0.01).
The results of Bonferroni’s post-hoc test. BMD: Bone mineral density. A, BMD L2-L4, B, BMD femur neck, C, BMD trochanter, D, BMD Ward’s triangle. a, P ≤ 0.01 significant difference compared to the male swimmers. b, P ≤ 0.01 significant difference compared to the female swimmers. c, P ≤ 0.01 significant difference compared to the male non-athletes. d, P ≤ 0.01 significant difference compared to the female non-athletes
Box plots of (A), L2-L4 T-scores, (B), Femur neck T-scores. Osteopenia: T-scores -1 to - 2.5 standard deviations (SDs). Osteoporosis: T-scores < - 2.5 SDs. a, P ≤ 0.05 significant difference compared to the male swimmers. b, P ≤ 0.05 significant difference compared to the female swimmers. c, P ≤ 0.05 significant difference compared to the male non-athletes. d, P ≤ 0.05 significant difference compared to the female non-athletes
5. Discussion
The first goal of this study was to compare the BMD values (proximal femur and lumbar spine) of elite swimmers with NA and to compare the corresponding values in elite MS and FS. It was hypothesized that swimming is neutral or ineffective on BMD improvement (22), and MS may have better bone acquisition than FS (12). However, the findings did not confirm these hypotheses; in general, it was shown that elite swimmers have better bone health than NA, and FS have slightly higher bone acquisition than MS, especially in the femoral neck area (except in the BMD of trochanter and Ward’s triangle). Detailed aspects of these results are discussed below.
The initial focus of this study was the comparison of bone condition between swimmers and NA. The findings showed that BMD values of swimmers in the proximal femur and lumbar spine areas were higher than NA. Also, examination of T-scores of 2 parts with high risks for fractures (femur neck and lumbar) showed that the average scores of swimmers in the lumbar area were -0.43 (males) to 0.51 (females) SDs, while the same scores for NA were - 0.78 (males) to - 0.69 (females) SDs. The superiority of swimmers (only females, not males) was also seen in the femur neck area (MS = - 0.55, FS = 0.53, male NA = - 0.50, and female NA = - 1.42 SDs).
Thus, professional swimming can be an effective activity in at least preventing bone loss and osteoporosis progression. Since very few studies have been performed on the bone health of elite swimmers (5, 18, 23, 24), the main findings of the present study are compared with the studies on non-professional swimmers; most of their results are inconsistent with this study (10, 18, 22, 23, 25), but there are also some compatible studies (5, 7, 9).
Gomez-Bruton et al., in a systematic review and meta-analysis, found that the swimmers have almost the same values of BMD as NA in the whole-body, femoral neck, and lumbar spine, which is in contrast with the findings of the present study (22). Another systematic review and meta-analysis in 2020 by Su et al. introduced swimming as a relatively effective activity to improve the bone density of postmenopausal women. They also proved that the swimmers who practice for long periods (3 to 6 hours a week or even longer) have better bone health than swimmers whose training time is less than 3 hours a week (7). Thus, their results are completely consistent with the findings of the present study; the possible reason could be the long period of training because the swimmers of the present study had a training time of 11 to 14 hours per week.
Ferry et al. showed that swimmers could not have a better bone condition in any body area than NA despite receiving calcium. This finding raises some questions. In people with adequate dietary intake, calcium supplementation without physical activity cannot induce any bone growth. Thus, the food consumption of those swimmers may have caused them to have no calcium need. On the other hand, the swimmers in this research were non-professional with fewer training hours (5 days/week, 10 hours/week), and they were also younger (15.9 ± 2 years); therefore, it is not possible to show the long-term effects of swimming in youth (18). These factors may have caused the conflicts in the results of the cited study with the present research. In contrast, in a recent cross-sectional study, Gheitasi et al. compared the BMD of young elite swimmers and NA and reported that although swimming is not osteogenic compared to weight-bearing sports, it is completely effective in improving bone density of the lumbar and femur regions of swimmers compared to NA. This finding is completely in accordance with the results of the current research, which can be due to the presence of subjects in contesting levels and championships; the type, intensity, and training volume of the swimmers in the two studies were similar (24).
As the second focus of the present study, it was hypothesized that swimming exercises have different effects on subjects of different sexes. The comparison of skeletal status in the lumbar and femur neck areas of the subjects revealed considerable results, showing that the mentioned areas in FS have relatively higher T-scores than MS. There is almost no study reporting findings consistent with these results; still, a few researchers proved the better adaptation of males’ bone tissue to swimming-related loads in comparison to females (11, 12), but some of them found no difference in the bone variables of swimmers of different sexes (11, 17, 26).
Therefore, in this respect, our findings contradict the findings of Ribeiro-Dos-Santos et al. These researchers had a 9-month follow-up study on adolescent swimmers and found that the boys acquired more BMD values than girls (8.47% in boys vs. 4.32% in girls) in the whole body and lumbar area. They even stated that practicing swimming for a long time may have negative effects on bone health (12). The difference in participants (adolescents), the type of study (cohort), or the length and duration of training (9 to 11 hours per week for 9 months) may be the potential reasons for the inconsistency of their results with the findings of the present study.
In another contrary study, Magkos F. et al. compared the bone density of the arm, femur, and trunk areas in water polo athletes, swimmers, and NA. They reported the bone density decline of the femur area in aquatic athletes (water polo and swimming) in comparison to NA, but no difference was seen in the athletes of different sexes (11). The lack of T-score evaluation based on age and sex in their study can possibly justify the opposite results of the two studies (13, 21). As mentioned before, the BMD of the femur neck area in the FS of the present study was quite prominent when T-scores were calculated, but when the BMD value of that area was compared alone, it did not seem notable.
Thus, as noted before, two important clinical outcomes can be observed from the findings of the current research. The first one is the superiority of swimmers to NA in gaining the BMD; it means that the swimmers gain more BMD than NA. While weight-bearing sports may increase the risk of fractures and cerebrovascular and cardiovascular diseases in people with osteopenia and osteoporosis, swimming can be the best option as a non-weight-bearing sport. It can be at least considered as a way of preventing the progression of osteoporosis (24). The second outcome is the better condition of female swimmers’ BMD than their male counterparts. The lifetime fracture risk in women over 50 years old is estimated at 50%, but this rate is about 20% in men; that is, the risk of fracture in women is about 2.5 times higher than in men. It can be a result of hormonal and skeletal differences between males and females, which make a difference in the bone’s response to physical activity. The insulin-like growth factor 1 and growth hormone have been proposed as the main determinants of sex differences in bone growth (27); in males, both estrogen and androgens stimulate periosteal bone expansion, which leads to cortical bone growth, but in females, estrogen stimulates endocortical apposition but limits periosteal bone expansion. The interactions between insulin-like growth factor 1, sex hormones, and training loads may be the reasons for the difference in bone acquisition between males and females (28). On the other hand, in females, the thickness of the cortex increases through the arrangement of endosteal bone without periosteal development, so it decreases the endosteal perimeter, whereas, in males, the changes in bone shape during its development are made by the expansion of periosteum size and the thickness of the cortex (13).
In summary, according to the findings of this study, competitive swimming may have positive effects on osteoporosis due to the following factors: First, swimming increases the levels of testosterone, estradiol, and sex hormones in the blood, which increase the bone matrix and stimulate the osteoblast cells (29). Second, this sport increases blood circulation in the body, resulting in the delivery of nutrients to the bone cortex, which affects the osteogenic process (30). Lastly, swimming can increase gastrointestinal peristalsis and vitamin D formation by absorbing calcium into the bone through the circulatory system (29). In general, because of the differences in hormonal mechanisms, the magnitude or detection of produced strains, body composition, response to mechanical stimuli, or other factors, the available evidence about the effect of physical activity in general and swimming in particular on the bone density acquisition of different sexes is very limited. Consequently, for an accurate answer to this question, more extensive research seems essential (11, 12, 17).
The present study had some limitations.
1. The researchers were unable to follow up on the subjects for a long period as the study was cross-sectional (11).
2. Since the swimmers were only the Iranian national team members, it was not possible to access more top-level or first-class athletes (only 14 subjects per group. A larger sample increases the statistical power.
3. The lack of precise control over the nutrition, diet, and hormonal status of the participants could help control the nutritional and endocrine confounders (31).
4. There was a lack of information about the swimmers’ training loads in various seasons, as the preparation techniques are confidential for each international coach. More information about the type, volume, and intensity of training could help present more accurate results (5).
The present study also had several strengths.
1. Since the swimmers were first-class competitors, researchers obtained the best results because these swimmers had regular exercises with high intensity, volume, and repetition (5).
2. As the BMD values of the participants were reported separately on the basis of sex, it is easy for the readers to recognize the exact effects of swimming on the bone condition of males and females (12).
3. The selection of swimmers and NA was made from people in a particular age range. They had passed growth spurts and puberty and had greater stability in their sexual hormones and other interfering factors (2, 29).
5.1. Suggestion
It is highly recommended that future studies focus on the different sexes of athletes in other popular aquatic exercises, such as recreational and rhythmic swimming while keeping the limitations of the present study in mind. Moreover, it seems essential that further studies determine whether different sexes have the same responses to bone acquisition.
5.2. Conclusions
In summary, elite swimmers have relatively better BMD values in two areas with high risks for fractures (L2-L4 and proximal femur) in comparison to their NA counterparts. Additionally, the FS have relatively better BMD values in the mentioned areas, especially in the femur neck (except for BMD in the trochanter and Ward’s triangle) than MS. Therefore, based on the results of this study, professional swimming can be useful for improving BMD, especially in females, and it can be considered very effective, at least in preventing the progression of osteoporosis.