1. Background
Elevated inflammatory biomarkers are important risk factors for age related morbidity and chronic diseases (1). Pro-inflammatory cytokines play a central role in immune responses. Furthermore, pro-inflammatory cytokines may be related to atherosclerosis, insulin resistance, and hypertension (2). According to previous studies, physical activity induces anti-inflammatory effects and reduces the risks of inflammatory related disease (3). Moreover, participants that tend to be active are at less risk of being diagnosed with chronic diseases than those that are not active or less active (4).
Resistance training (RT) is defined as the static or dynamic contractions of muscle against external resistances with different intensities. Long-term health benefits of RT have been presented previously (5). Systematic RT leads to the increase in muscle strength, endurance, and mass. Moreover, in addition, RT is related to the reduction in low grade inflammation related diseases, such as type 2 diabetes and cardiovascular diseases (6).
Unlike chronic exercise, a heavy training session may cause transient increases in inflammation (7). However, this finding is inconsistent, and the increases range from mild to intense (7, 8). This controversy may be related to different factors including differences in the characteristics of the participants, exercise type and intensity, intervention period, sampling time, genetic differences, and the method used to estimate circulatory cytokine concentrations. Furthermore, training experience may also have an effect on metabolic and inflammatory responses. Few studies are available regarding the comparison of metabolic and inflammatory responses in trained and untrained people. The present study tests two hypotheses. First, we hypothesize that metabolic, inflammatory and muscular damage response during a resistance exercise will be significantly different between trained and untrained subjects. Second, we hypothesize that the different response will be associated with body composition and the weight that each subject lifted.
3. Methods
This clinical trial was conducted on 28 men with normal body compositions (Percentage Body Fat = PBF < 25%) (9). Subjects were screened according to the inclusion and exclusion criteria. Inclusion criteria were: 20 - 30 years of age, non-smoker, no alcohol usage, no usage of dietary or sports supplements, and with no weight changes in the past 6 months. Exclusion criteria were: history of inflammatory and chronic disease and using drugs which have an effect on metabolism, glucose profile, and inflammatory status.
3.1. Study Design
Twenty eight young adults (24.35 ± 2.3 years old, 176.42 ± 6.8 cm height, 78.2 ± 6.3 kg weight, and BMI = 25.11 ± 1.4 kg/m2) were included in the study. The primary screening was conducted by a phone call after a brief explanation about the study’s procedures and primary assessment. The final screening was performed according to the inclusion and exclusion criteria. The inclusion criteria were limited to subjects with an experience of at least 2 years of bodybuilding and with at least 3 training sessions per week (for trained participants). The untrained group consisted of subjects without exercise activity during at least the past 2 years. All subjects followed at least 80% of the dietary guidelines for Americans 2010 (10). Participants consuming more than 300 mg of caffeine daily (described as caffeine users) were excluded from the study (11). Because of the adverse relationship between exercise performance and/or hormonal levels and sleep disorders, subjects having any kind of sleep disorders and/or sleep loss were excluded (12, 13). Therefore, subjects who slept 7 - 8 hours at any time during the 24-hour day were included in the study. The selected participants were divided into two groups, either into the trained or the untrained groups after signing an informed consent.
3.2. Resistance Training Protocol
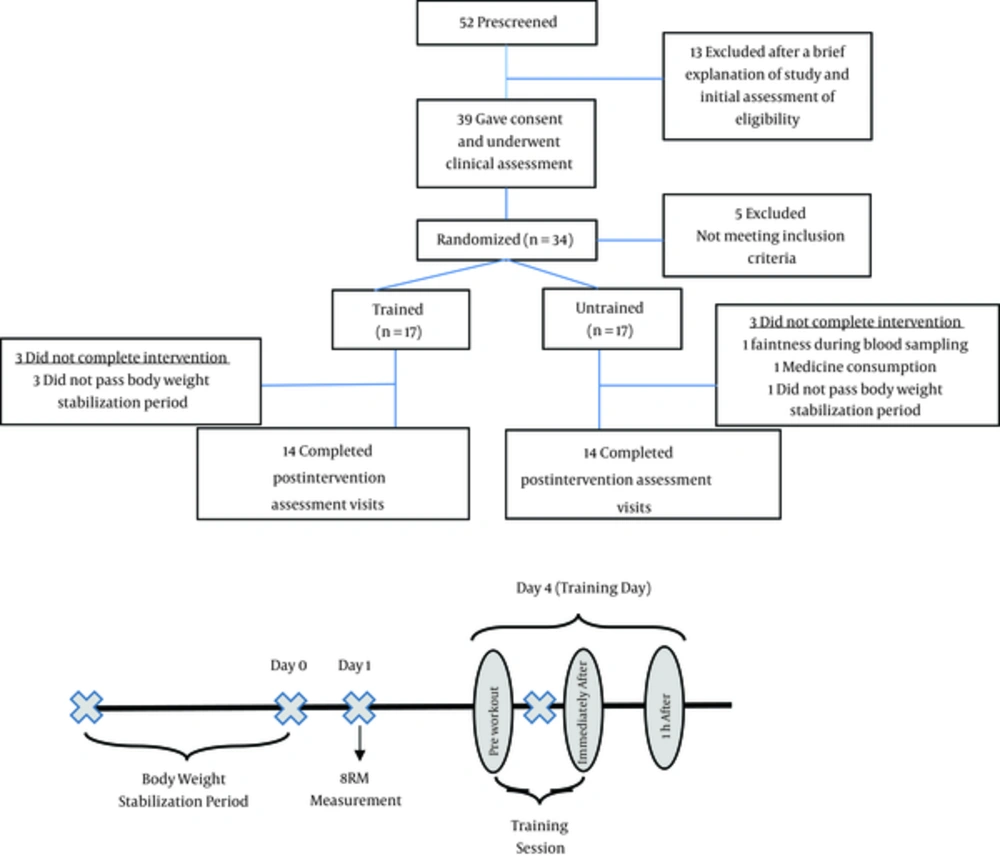
Prior to exercise intervention, an ambulatory run-in period was imposed for each subject to ensure the stabilization of the body weight (± 2 kg change during 4 weeks). The subjects were instructed to refrain from vigorous physical activity for 48 hours before exercise sessions. After body weight stabilization, 8-repetition maximum (8RM) was measured for each training exercise (14). A training session including 9 upper body (chest and arms) exercises was conducted for each of the athletes, three days after 8RM measurement. The movements included barbell bench press, dumbbell bench press, incline barbell press, incline dumbbell fly, cable crossover, barbell curl, incline dumbbell curl, lying triceps extension, and close-grip bench press. Three sets of each movement was performed and with 8 repetitions. The diagrams of study have been presented in Figure 1.
Because of the diet’s important role in metabolism and systematic inflammation (15), dietary intake has been equalized during a training day. This equalization performed based on nutrient timing principles. The basis of nutrient timing involves the consumption of combinations of macronutrients in and around an exercise session based on the body weight (16). Table 1 has presented the homogenization protocol.
| Variables | Amount, g/kg | Food items | Time |
|---|---|---|---|
| Waking up | - | - | 9:00 am |
| Breakfast | Carbohydrate: 2 | Bread, low-fat cheese, honey, fruit (apple), low-fat milk | 9:30 am |
| Protein: 0.2 | |||
| Fat: 0.1 | |||
| Pre-exercise meal | Carbohydrate: 3 | Rice, chicken breast, low-fat, yogurt, oil | 12:00 (noon) |
| Protein: 0.6 | |||
| Fat: 0.2 | |||
| Pre-exercise snack | Carbohydrate: 1 | Fruit (banana) | 14:00 |
| Protein: - | |||
| Fat: - | |||
| Training session | - | - | 16:00 |
The Amount of Macronutrient Intake in Exercise Days
3.3. Anthropometric and Blood Pressure Measurement
Body composition was assessed by using the direct segmental multi-frequency bioelectrical impedance (inbody 270, Biospace, Korea) at fasting and euhydrated state. All anthropometric measures were duplicated, and means were reported for each of the participants.
3.4. Blood Pressure Measurement
The participants were asked to sit on a chair and have no physical activity 1 hour before sampling. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and the heart beats were measured, 10 minutes before and immediately (15 to 30 seconds) (17)) and 1 hour after training by an automatic blood pressure monitors (Jawon Medical, Korea). The mean arterial pressure (MAP) was calculated using the formula: (2DBP + SBP)/3. On the other hand, the rate pressure product (RPP) was calculated using HR multiplied by SBP and pulse pressure using the differences between SBP and DBP (SBP-DBP).
3.5. Biochemical and Inflammatory Analysis
Approximately 5 ml of peripheral blood was taken from the subjects 15 minutes before, immediately and 1 hour after RT. Blood glucose concentrations, creatine kinase (CK), and lactate dehydrogenase (LDH) activities were measured using the spectrophotometry method (Pars Azmoon Inc., Iran) by an auto-analyzer (Hitachi, USA). Furthermore, the plasma insulin concentration was measured using the ELISA method (Diaplus Inc., Canada). In addition, the HOMA-IR index has been calculated by using the (FBS (mg/dL)*fasting insulin (insulin (μU/mL))/405 formula (18), while the secreted cytokines concentrations in samples were measured using the sandwich ELISA method (Eastbiopharm, China).
3.6. Statistical Analysis
A repeated measures test was used to compare variables before, immediately, and 1 hour after training. To find significant differences between these variables, the post hoc test (LSD) was used. Normality of data was assessed using Kolmogorov-Smirnov test. A subject’s correlation coefficients were used to examine the relationships between exercise intensity and changes in each of the baseline anthropometric measures, metabolic, inflammatory, and blood pressure parameters during exercise. In addition, P < 0.05 was considered statistically significant. All of the data was expressed as mean values ± standard deviation (SD). Statistical analysis was done using the statistical package for social sciences (SPSS) for windows version 19 (SPSS Inc., Chicago, IL).
4. Results
Baseline anthropometric measures and training characteristics of the subjects in both the trained and untrained groups are presented in Tables 2 and 3, respectively. As shown in Table 2, only percent lean body mass in the trained group was significantly more than the untrained group. According to Table 3, all of the training variables have significant differences between two groups, except for the training duration. This means that participants with more training experience have lifted heavier weights. Based on Table 4, which shows the baseline biochemical and inflammatory status in the participants, CK, heart rate (HR), and pulse pressure have significant differences between the two groups. Results demonstrated that the CK and pulse pressures were higher, while HR was less in the trained group.
| Variables | Baseline (Untrained) N = 14 | Baseline (Trained) N = 14 | P Value |
|---|---|---|---|
| Age, y | 23.57 ± 1.3 | 25.14 ± 2.9 | 0.078 |
| Weight, kg | 78.45 ± 6.8 | 77.95 ± 5.9 | 0.839 |
| Height, cm | 177.88 ± 8.9 | 174.95 ± 3.4 | 0.265 |
| BMI, kg.m-2 | 24.78 ± 1.0 | 25.45 ± 1.7 | 0.226 |
| LBM, kg | 36.19 ± 3.7 | 37.28 ± 3.3 | 0.42 |
| FM, kg | 14.95 ± 2.7 | 13.1 ± 3.0 | 0.107 |
| TBW, kg | 46.57 ± 4.5 | 47.54 ± 3.7 | 0.543 |
| FFM, kg | 63.44 ± 6.2 | 64.79 ± 5.1 | 0.539 |
| PBF, % | 19.11 ± 3.1 | 16.77 ± 3.4 | 0.071 |
| PLBM, % | 46.1 ± 1.9 | 47.83 ± 2.2 | 0.04 |
| WC, cm | 84.19 ± 3.5 | 83.17 ± 4.2 | 0.5 |
| HC, cm | 96.71 ± 5.5 | 96.82 ± 3.4 | 0.952 |
Basic Anthropometric Characteristics of Study Subjectsa
| Variables | Baseline (Untrained) N = 14 | Baseline (Trained) N = 14 | P Value |
|---|---|---|---|
| Barbell bench press, kg | 65.71 ± 5.1 | 84.64 ± 16.5 | < 0.001 |
| Dumbbell bench press, kg | 30.35 ± 6.3 | 51.42 ± 11.6 | < 0.001 |
| Incline barbell press, kg | 41.78 ± 5.7 | 63.57 ± 16.8 | < 0.001 |
| Incline dumbbell fly, kg | 17.5 ± 4.2 | 26.07 ± 4.8 | < 0.001 |
| Cable crossover, kg | 33.57 ± 7.7.4 | 46.42 ± 11.5 | < 0.001 |
| Barbell curl, kg | 23.92 ± 4.8 | 34.28 ± 3.8 | < 0.001 |
| Incline dumbbell curl, kg | 17.5 ± 4.2 | 26.42 ± 3.6 | < 0.001 |
| Lying triceps extension, kg | 23.21 ± 3.1 | 33.92 ± 6.5 | < 0.001 |
| Close-grip bench press, kg | 40.0 ± 7.0 | 64.28 ± 16.2 | < 0.001 |
| All workouts, kg | 293.57 ± 30.4 | 431.42 ± 74.3 | < 0.001 |
| Duration of training, min | 41.28 ± 4.9 | 38.14 ± 4.7 | 0.1 |
Workout Characteristics of Study Subjectsa
| Variables | Baseline (Untrained) N = 14 | Baseline (Trained) N = 14 | P Value |
|---|---|---|---|
| BS | 94.59 ± 8.65 | 93.66 ± 12.1 | 0.817 |
| insulin | 18.84 ± 10.2 | 13.42 ± 8.58 | 0.141 |
| CK | 157.9 ± 49.6 | 267.43 ± 160.6 | 0.022 |
| LDH | 267.23 ± 18.2 | 285.35 ± 33.6 | 0.088 |
| SBP | 111.71 ± 10.3 | 118.21 ± 8.6 | 0.084 |
| DBP | 76.07 ± 7.7 | 72.78 ± 8.1 | 0.284 |
| HR | 82.14 ± 11.0 | 70.85 ± 8.5 | 0.005 |
| RPP | 9140.28 ± 1205.8 | 8351.42 ± 979.2 | 0.069 |
| Pulse pressure | 35.64 ± 9.1 | 45.42 ± 5.1 | 0.002 |
| MAP | 87.95 ± 7.5 | 87.92 ± 7.9 | 0.994 |
| HOMA-IR | 4.49 ± 2.6 | 3.21 ± 2.1 | 0.174 |
| HOMA-B | 218.72 ± 132.8 | 161.52 ± 100.7 | 0.211 |
| IL-6 | 0.5 ± 0.2 | 0.42 ± 0.25 | 0.401 |
| TNF-α | 0.49 ± 0.29 | 0.44 ± 0.25 | 0.689 |
| IL-10 | 0.3 ± 0.16 | 0.31 ± 0.2 | 0.938 |
| IL-6/IL-10 ratio | 1.89 ± 0.99 | 1.51 ± 0.45 | 0.207 |
| TNF-α /IL-10 ratio | 1.57 ± 0.29 | 1.58 ± 0.4 | 0.902 |
Basic Biochemical and Inflammatory Characteristics of Study Subjectsa
Biochemical and inflammatory changes in the training session are shown in Table 5. Although, DBP, SBP, RPP, MAP, and IL-6 were significantly different between the trained and untrained groups, insulin concentration, BS, HOMA-IR, HOMA-B, CK, LDH, HR, pulse pressure, IL-10, IL-6/IL-10 ratio, TNF-α, and TNF-α /IL-10 ratio did not show significant differences. Insulin concentration and HOMA-IR were significantly reduced immediately, and were compared 1 hour after training with results of the state before training. Similarly, the BS concentration was reduced significantly in the untrained group. However, BS reduction was not found in the trained group. Reduction in HOMA-IR was also significant between 1 hour after and immediately after training states.
| Variables | Pre workout | Immediately after | 1 hours after | Intra group P | Between group P | |
|---|---|---|---|---|---|---|
| insulin | Un | 18.84 ± 10.2 | 11.25 ± 5.5b | 10.44 ± 7.3b | 0.015 | 0.597 |
| Tr | 13.42 ± 8.5 | 9.0 ± 5.3b | 5.93 ± 2.1c,d | 0.002 | ||
| BS | Un | 94.59 ± 8.6 | 88.9 ± 6.6b | 82.49 ± 4.6c,d | < 0.001 | 0.615 |
| Tr | 93.66 ± 12.1 | 91.89 ± 12.6 | 85.69 ± 4.1 | 0.132 | ||
| HOMA-IR | Un | 4.49 ± 2.6 | 2.52 ± 1.3b | 2.12 ± 1.4b | 0.007 | 0.6 |
| Tr | 3.21 ± 2.1 | 1.99 ± 1.0b | 1.25 ± 0.4b,d | 0.003 | ||
| HOMA-B | Un | 218.72± 132.8 | 155.47 ± 58.1 | 203.34 ± 160.3 | 0.369 | 0.271 |
| Tr | 161.52 ± 100.7 | 138.63 ± 126.1 | 97.36 ± 38.3 | 0.103 | ||
| CK | Un | 157.9 ± 49.6 | 212.57 ± 60.9c | 236.02 ± 111.8c | 0.006 | 0.075 |
| Tr | 267.43 ± 160.6 | 374.5 ± 231.0c | 385.15 ± 242.8c | 0.001 | ||
| LDH | Un | 267.23 ± 18.2 | 302.61 ± 28.8c | 284.76 ± 31.2b,e | < 0.001 | 0.749 |
| Tr | 285.35 ± 33.6 | 321.69 ± 43.9c | 298.42 ± 49.9e | < 0.001 | ||
| SBP | Un | 111.71 ± 10.3 | 115.28 ± 9.2 | 110.28 ± 6.9 | 0.223 | < 0.001 |
| Tr | 118.21 ± 8.6 | 134.64 ± 9.6c | 109.0 ± 11.0c,e | < 0.001 | ||
| DBP | Un | 76.07 ± 7.7 | 76.21 ± 12.7 | 80.14 ± 11.0 | 0.383 | 0.015 |
| Tr | 72.78 ± 8.1 | 81.64 ± 12.7b | 71.07 ± 9.1d | 0.009 | ||
| HR | Un | 82.14 ±11.0 | 111.5 ± 10.7c | 84.85 ± 7.7e | < 0.001 | 0.63 |
| Tr | 70.85 ± 8.5 | 111.92 ± 15.3c | 76.71 ± 11.9e | < 0.001 | ||
| RPP | Un | 9140.28 ± 1205.8 | 12915.28 ± 2113.1c | 9351.57 ± 999.84e | < 0.001 | 0.001 |
| Tr | 8351.42 ± 979.2 | 15088.64 ± 2467.9c | 8296.14 ± 1062.9e | < 0.001 | ||
| Pulse pr | Un | 35.64 ± 9.1 | 39.07 ± 12.0 | 30.14 ± 12.3d | 0.048 | 0.416 |
| Tr | 45.42 ± 5.1 | 53.0 ± 13.5b | 37.92 ± 10.9b,d | 0.001 | ||
| MAP | Un | 87.95 ± 7.5 | 89.23 ± 10.1 | 90.19 ± 7.9 | 0.691 | 0.001 |
| Tr | 87.92 ± 7.9 | 99.3 ± 9.9b | 83.71 ± 8.3e | < 0.001 | ||
| IL-6 | Un | 0.5 ± 0.2 | 0.58 ± 0.25b | 0.72 ± 0.26c,d | 0.004 | 0.019 |
| Tr | 0.42 ± 0.25 | 0.54 ± 0.28c | 0.5 ± 0.26b | 0.004 | ||
| TNF-α | Un | 0.49 ± 0.29 | 0.51 ± 0.28 | 0.51 ± 0.29 | 0.314 | 0.768 |
| Tr | 0.44 ± 0.25 | 0.49 ± 0.27 | 0.47 ± 0.26 | 0.175 | ||
| IL-10 | Un | 0.3 ± 0.16 | 0.34 ± 0.2 | 0.34 ± 0.21 | 0.122 | 0.088 |
| Tr | 0.31 ± 0.2 | 0.29 ± 0.19 | 0.26 ± 0.16 | 0.29 | ||
| IL-6/IL-10 ratio | Un | 1.89 ± 0.99 | 1.98 ± 0.64 | 2.91 ± 2.26 | 0.117 | 0.469 |
| Tr | 1.51 ± 0.45 | 2.17 ± 0.75 | 2.42 ± 1.68 | 0.124 | ||
| TNF-α /IL- 10 ratio | Un | 1.57 ± 0.29 | 1.64 ± 0.51 | 1.62 ± 0.47 | 0.809 | 0.223 |
| Tr | 1.58 ± 0.4 | 1.86 ± 0.54 | 2.2 ± 1.4 | 0.069 | ||
Biochemical and Inflammatory Changes During Exercise Sessiona
Both of CK and IL-6 concentrations increased immediately and 1 hour after training compared with the state before training. Also, IL-6 concentration showed a significant increase 1 hour compared with immediately after training. In addition, LDH, RPP, and HR significantly increased immediately after training, and reduced significantly 1 hour after training compared with the state before training. Similarly, MAP significantly increased immediately and reduced 1 hour after being compared with the state before training in the trained group. Significant increases in the LDH concentration were also found in the untrained group, 1 hour after compared with the states before training.
SBP and DBP significantly increased immediately after training and then reduced 1 hour after training compared with the state before training in the trained group. The pulse pressure in the trained group increased immediately after being compared with the results before training. However, it significantly reduced 1 hour compared to immediately the state after training.
According to Pearson’s correlation test, heavier weight lifting was positively correlated to muscle mass percentage, CK activity, and SBP. Moreover, reduction in blood sugar during training was positively correlated to insulin and HOMA-IR changes and negatively to PLBM. Changes in IL-6 after RT were inversely associated to baseline PBF and WC. Increases in HR after RT were significantly and positively in relation with blood sugar, insulin, and HOMA-IR changes (Table 6).
| Variables | BMI | PLBM | PBF | WC | Intensity | ΔIL-6 | ΔCK | ΔLDH | ΔBS | Δ Insulin | ΔHOMA-IR | ΔSBP | ΔHR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | 1 | ||||||||||||
| PLBM | 0.51b | 1 | |||||||||||
| PBF | 0.32 | -0.49b | 1 | ||||||||||
| WC | 0.41c | 0.16 | 0.63b | 1 | |||||||||
| Intensity | 0.34 | 0.38c | -0.35 | -0.26 | 1 | ||||||||
| ΔIL-6 | -0.20 | 0.04 | -0.43c | -0.46c | -0.09 | 1 | |||||||
| ΔCK | 0.26 | 0.3 | -0.27 | -0.16 | 0.76b | -0.12 | 1 | ||||||
| ΔLDH | 0.21 | -0.13 | 0.14 | -0.05 | 0.31 | -0.14 | 0.64b | 1 | |||||
| ΔBS | -0.35 | -0.6b | 0.2 | -0.19 | 0.01 | 0.04 | -0.15 | 0.08 | 1 | ||||
| Δ Insulin | 0.06 | -0.11 | 0.1 | -0.15 | 0.29 | -0.06 | 0.04 | 0.04 | 0.53b | 1 | |||
| ΔHOMA-IR | 0.004 | 0.18 | 0.18 | -0.14 | 0.24 | -0.07 | 0.01 | 0.06 | 0.65b | 0.98b | 1 | ||
| ΔSBP | -0.32 | 0.7 | -0.5b | -0.53b | 0.42c | 0.21 | 0.13 | 0.03 | 0.31 | 0.14 | 0.14 | 1 | |
| ΔHR | -0.2 | -0.29 | 0.03 | -0.2 | 0.33 | -0.09 | 0.07 | -0.06 | 0.63b | 0.68b | 0.73b | 0.31 | 1 |
Pearson Correlation Among Anthropometric Values and Metabolic Valuesa
5. Discussion
The results of our study have shown that one training session causes metabolic and inflammatory changes. So that, a RT session has significantly reduced insulin and HOMA-IR in both of the groups, immediately and 1 hour after training compared with the state before training. Moreover, BS concentrations have been significantly reduced in the untrained group. According to these findings, a single RT session, whether in trained or untrained men could reduce insulin concentrations and insulin resistance and modify glucose concentrations in untrained men. Between the groups, comparison did not show a significant difference in responses between the trained and untrained subjects.
Magkos et al. have reported that a RT session not only improves insulin resistance by HOMA-IR reduction, but also reduces insulin and blood glucose concentrations (19). Another study has shown insulin resistance reduction during 2 hours after training (20). It has been suggested that this reduction in insulin resistance is in relation with energy expenditure during training (19). As previously mentioned, skeletal muscle mass is in charge of insulin dependent glucose uptake in the human body. Insulin dependent glucose uptake capacity is directly related to muscle mass and inversely to fat mass (21). Based on our study, blood glucose reduction during training has been strongly and positively associated with muscle mass percentage. On the other hand, during RT, in the subjects with more muscle mass there was more reduction in blood glucose.
On the contrary, numerous studies on endurance exercise have shown that this type of exercise also improves insulin resistance in young and old subjects (22). On the other hand, even one session moderate to intense endurance training clearly improves insulin sensitivity or glucose resistance (21). GLUT-4 protein express has an important role in insulin dependent glucose uptake of skeletal muscles. It seems that an increase in this protein gene expression, mainly in long-term training may decrease insulin resistance. Improvements in insulin resistance during a session of body building are mainly related to increases in GLUT-4 translocation (23).
Athletes have higher CK concentrations in the resting state, which may be related to a higher muscle mass and daily training (24). This finding is in line with our study results (Table 4). Based on our study, there was a positive and significant correlation between percent muscle mass and baseline CK concentration (P = 0.014, r = 0.458). CK and fat mass percent were positively and significantly correlated (P = 0.011, r = 0.471) (data not shown). On the other hand, CK baseline activity increased by muscle mass increases and decreases in fat mass percent. This significant correlation was not found between body composition indexes including LDH. CK and LDH isoenzymes are muscle injury biomarkers (25). Some studies have shown changes in muscle enzymes and isoenzymes serum concentrations after intense training in athletes and untrained subjects (26, 27). Increased CK activity may be due to metabolic and mechanical causes. Another mechanism may be due to local tissue damage by sarcomere deterioration in the Z-line (27). The present study also found significant increases in CK activity in both groups, immediately and 1 hour after training compared with the state before training. Although, CK was increased even 1 hour after training, LDH activity was reduced after training. LDH activity has significantly increased immediately after training in both of the groups, and decreased 1 hour after being compared with the state immediately after training. Although, LDH was reduced 1 hour compared with the state immediately after training, it reached the baseline values only in the training group. After 1 hour of training, LDH activity was still significantly higher than baseline in the untrained group. This difference between the two groups may be related to higher muscle damage in the untrained subjects and better muscle accordance to RT in the trained subjects. Based on our findings, CK activity increases are significantly related to lifted weights’ heaviness. On the other hand, higher weight lifting induces increases in serum CK activity. Although, baseline CK activity is related to muscle mass, increases in CK activity following RT did not show a significant relation with the muscle mass, and was only due to higher weight lifting in the trained group (Table 6).
Similar to our study, Rodrigues et al. have investigated muscle damages during two sessions of RT. CK activity had increased after both of the trainings and was increasing up to 48 hours, and then decreased 72 hours after training. However, CK activity after 72 hours of training was still higher than baseline (28). Guzel et al. have investigated two RT interventions with different intensities (low and high intensity) effects on muscle damage factors, and reported increases in CK activity immediately after RT which remained up to 24 hours after training, and then reduced (29). Previously, the highest CK activity has been reported in less trained subjects (30), which is in contradiction with our results. Based on our findings, there was a 34.6% increase in the CK activity in the untrained group immediately after being compared to the state before training; however, this was 40.1% in the trained group. Although, this difference was not significant, it may be related to higher weights lifted by the trained subjects. Furthermore, CK increased significantly 1 hour compared to immediately after training 11% and 2.8% in the untrained and trained subjects, respectively (data not shown). This indicates that despite higher increases of CK in the trained subjects during training, these increases diminished during 1 hour of training probably show a better accommodation of muscle mass to decrease muscle damages in the trained group.
Exercise has beneficial effects on cardiovascular health. Recently, additional attention was given to one session of training’s beneficial effects on cardiovascular system along with systematic training. After training, blood pressure reduced within minutes after training and remained at low levels of baseline for hours. This phenomenon is called post-exercise hypotension (PEH) (31). Previously, it has been shown that PEH is occurred either by continuous dynamic training such as running and cycling or intermittent training such as resistance exercise (32). PEH increased to higher levels in the trained group in our study. On the other hand, all measured blood pressure parameters in the trained group changed significantly during different periods; however, these changes were only significant for HR, RPP, and the pulse pressure in the untrained group. Since, factors could make these response differences such as the study population (normotensive or hypotensive), exercise intensity and modality, and exercise duration was similar between the two groups (32), it seems that these differences are due to the differences in the heaviness of weights lifted by the trained and untrained groups (33). The results emphasize that SBP (not HR) response during one session of RT was significantly related to lifted weight’s heaviness. On the other hand, since the trained subjects lifted heavier weights, they have higher SBP response. Since SBP has important roles on all indexes of the study (RPP, MAP, and pulse pressure), these differences at 1-RM and training history, which have caused differences in SBP, may be the major cause of differences in cardiovascular responses. It has been well illustrated that RPP, which is an indirect index for myocardial oxygen demand, is increased during RT sessions (5). Although RPP is gained from HR multiplied by SBP formula and these parameters remained unchanged in the untrained group, RPP increased immediately after training and returned to the before state 1 hour after training, similar to the trained group. Based on the mentioned reasons, cardiovascular responses in the present study are significantly different at the trained and untrained subjects, and they are more intensive in the trained compared with untrained subjects before, immediately after, and 1 hour after training.
Furthermore, among inflammatory parameters, IL-6 concentration has significantly increased in both of the two groups immediately and 1 hour after training compared to the state before training. Although, Il-6 tended to decrease in the trained group during 1 hour of training, which was statistically non-significant, it has significantly increased in the untrained group 1 hour after training. On the other hand, a more intense response was found at the untrained subjects. It seems that increases in IL-6 concentrations are due to muscle fibers contraction, which induces different metabolic effects (34).
Accumulated data has supported this hypothesis that IL-6 secretion, due to muscle contractions during training acts in a hormone like manner to mobilize extracellular substrates and/or augment substrate delivery during exercise (35). These increases in IL-6 concentration due to exercise, has stimulated lipolysis and fat oxidation and is involved in glucose hemostasis, especially by glycogenolysis induction (34, 35). Based on the mentioned information, although increases in Il-6 are due to muscle contractions, increases in this inflammatory marker have not been related to muscle mass in our study. Our results have shown that increases in IL-6 following RT are dependent on training intensity and lifted weights heaviness. Moreover, an inverse relation was found between the percentage of fat and the abdominal circumference with IL-6 increases during the training (Table 6). In line with our results, Philips et al. have investigated the effects of one session of bodybuilding training at different intensities (intense and light) in 14 recreationally active men without body building history, and have reported that IL-6 concentration increased immediately after training at the two groups and then returned to the baseline, after 6 hours (36). A study on 24 active women has found controversies. Based on this study’s results, there was no changes in serum concentrations of cytokines (Interleukin 1β, 2, 5, 6, 8, 10, and TNF-α); however, Interleukins 1β, 6, 8, and TNF-α mRNA had up-regulated (37).
The present study could not find any significant differences in other cytokines and inflammatory parameters between the two groups. This result may be due to the IL-6 biological role. According to this, increases in IL-6 concentration due to muscle contractions during intense exercise, has strong anti-inflammatory effects and could reduce TNF-α, inhibit TNF-induced insulin resistance, and increase anti-inflammatory factors, such as IL-1ra and IL-10 (34, 35). This indicates an anti-inflammatory role of IL-6, which is secreted from myocytes as a supportive response during a training session (6).
It has been shown that the balance between inflammatory and anti-inflammatory cytokines is important to the proper assessment of inflammatory responses (38), and this proportion explains the balance (39). Previously, it has been suggested that imbalances between inflammatory and anti-inflammatory effects are related to chronic disease vulnerability. Therefore, the measurement of proportions of inflammatory and anti-inflammatory factors indicate an absolute range of immunological status (40), which significantly increases in some diseases and unhealthy conditions (41) and cardiovascular diseases (42). Previously, it has been shown that systematic exercise could reduce TNF-α to interleukin-10 proportion (43). Ghafourian et al., in 2016 has reported that 1 training session significantly increases interleukin 6 to interleukin 10 proportion, however, TNF-α to interleukin 10 proportion was not statistically significant (44).
In summary, this study has shown that a session of strenuous RT indicates metabolic and inflammatory changes. This response can be different at trained and untrained subjects. After a session of RT, reduction in blood glucose and insulin, and improvement of insulin resistance were seen in both groups. Furthermore, RT has been associated with muscle damage and increases in muscle enzymes. Although RT can increase IL-6 concentration, as an inflammatory cytokine, inflammatory to anti-inflammatory cytokine proportions, as an inflammation marker, remained unchanged. Across the measured factors, there were significant differences in blood pressures and IL-6 responses, between the two groups.