1. Background
Transcutaneous electrical nerve stimulation (TENS) has been proposed for relieving pain due to musculoskeletal injury. The neurophysiological principle of pain relief from TENS is derived from the gate control theory in which non-nociceptive afferent fibers activate interneurons at the spinal cord level, which then inhibit the activity of nociceptive projection neurons. Transcutaneous electrical nerve stimulation selectively activates Aβ large-diameter afferent fiber by high-frequency stimulation (> 50 Hz, inhibiting constant transmission of nociceptive neurons (1-3). On the other hand, low-frequency stimulation (< 50 Hz) can activate the release of endogenous opioids and serotonin, which subsequently decrease muscle pain at the central nervous system (CNS) level (4). Although most clinical studies demonstrated the benefit of TENS for reducing pain intensity, many of them showed no benefits or inconclusion (5). The controversy could be due to varieties of the intervention protocol, the primary cause of pain, the level of initial pain, or the pain assessment techniques.
Various techniques have been used in evaluating conscious pain intensity. A simple pain scale is widely used; however, results are subjective, in which response bias or placebo effect might be involved. Apart from the pain scale, pain threshold measurement is more reliable. Two common types of measurement that have been accomplished are the pressure pain thresholds (PPT) and temperature pain threshold. Between the two types, the PPT has commonly been used in assessing musculoskeletal pain and myofascial pain (6). In a study with healthy volunteers, the PPT was significantly increased after high-frequency TENS treatment (7). Although previous studies revealed that PPT measurement has high reliability for musculoskeletal pain, raters with previous familiarization and practice are required (8). Additionally, although the PPT provided high intra-rater reliability, inter-rater and inter-subject are still questioned. For example, the PPT in trained athletes is usually higher than in untrained individuals (9). Therefore, the cuff-off values of the PPT might not be universal. Based on these disadvantages, alternative pain assessment methods are being investigated.
Heart rate variability (HRV) has been introduced for the screening of pain intensity. Heart rate variability is the variance in time between each heartbeat. It is measured by the variation in the beat-to-beat interval. Heart rate variability indexes neurocardiac function, which is generated by dynamic non-linear autonomic nervous system (ANS) processes (10). The data then provide measures of autonomic nervous activity in both sympathetic and parasympathetic signals (11). According to many involvements in autonomic control that are tightly shared with areas involved in pain perception (12), HRV is then considered a potential index of autonomic response to noxious stimuli. A recent systemic review concluded that HRV measurement demonstrated significant changes in both sympathetic and parasympathetic ANSs during painful stimulation independent of the pain induction method (13). Responses could be affected by several factors, such as gender, age, body mass index, breathing patterns, the intensity of the stimulation, and the affective state. Nevertheless, most of the previous studies were performed on healthy volunteers receiving exogenous pain stimulation. Therefore, the HRV information in true patients with musculoskeletal pain is inadequate to be concluded.
2. Objectives
Therefore, the present study aimed to evaluate whether HRV could be another parameter indicating pain score upon treatment. Both the time domain and frequency domain of HRV were determined before, during 30 minutes of TENS intervention, and thereafter in young athletes with musculoskeletal pain in the lower extremity. The correlation between PPT and HRV parameters was evaluated. In addition to the application of HRV in pain management, the information from the clinical study could provide a better understanding of the changes in autonomic response during electrical nerve stimuli.
3. Methods
3.1. Participations and Study Design
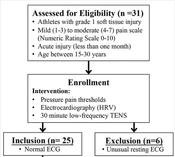
This study was a one-group quasi-experimental design in which 31 athletes with grade 1 soft tissue injury participated (Figure 1). The study was approved by the Central Institutional Review Board of Mahidol University in Nakhon Pathom, Thailand (MU-CIRB 2021/518.2812) in accordance with the Declaration of Helsinki. Before participation, all the participants were informed of the procedure and signed an informed consent. The participants were athletes who had at least one year of training experience with soft tissue acute injury at mild (score of 1 - 3) to moderate (score of 4 - 7) pain based on the Numeric Rating Scale (NRS) (score of 0 - 10) as described by By Forte et al. (13). The NRS scores ≤ 5, 6 and 7, and ≥ 8 correspond to mild, moderate, and severe pain-related interference with functioning, respectively.
The participants were excluded if they presented contraindications related to TENS, such as a history of epilepsy, presence of a pacemaker or metal implants, no chronic use of analgesic or anti-inflammatory drugs, and not undergoing a physical therapy session or muscle strengthening program for lower limb in the past 3 months. The participants were asked to refrain from taking analgesics a day before and during the day of the experiment. The participants were also asked to refrain from consuming caffeine and doing intense physical activity 24 hours before the experimental day. After collecting demographic data, the participants received a 30-minute TENS intervention, and the PPT and electrocardiography (ECG) were evaluated before and after the intervention. The PPT assessor was blinded to participants’ identification and their pain rating scores. Only one assessor performed all PPT measurements to limit intra-rater and inter-rater reliability.
3.2. Instruments
3.2.1. Transcutaneous Electrical Nerve Stimulation
A Transcutaneous Electrical Nerve Stimulation (TENS) device (model 1498920 Endomed 482, Enraf-Nonius, Netherland) with adhesive surface electrodes 5 × 5 cm was used in the study. The participants sat in a relaxing position. Low-frequency TENS (LF-TENS) at a frequency of 4 Hz with a pulse duration of 200 - 300 µs was performed for all participants. Before the procedure, the therapist explained the sensations from TENS stimulation that the participants might experience during treatment (e.g., tingling, tapping, buzzing, or muscle twitching). The skin around the pain point was gently cleaned with 70% ethyl alcohol before putting on two pairs of surface electrodes across the pain point. The stimulation intensity was gradually adjusted until it reached the subject’s perceived sensation point with muscle twitching. Transcutaneous electrical nerve stimulation therapy was continued for 30 minutes. Standard limb leads of ECG were also continuously measured before, during, and after the protocol. The PPT was measured before and after the treatment and 24 hours later. After the study, the participants received the TENS treatment for another 4 to 6 days at their convenience.
3.2.2. Pressure Pain Thresholds
The subjects were asked to rest for 5 minutes prior to starting with a baseline assessment of the PPT and ECG. Transcutaneous electrical nerve stimulation was started after PPT measurement for another 5-minute rest. The PPT was assessed by an algometer (force TENTM FPX digital algometer, Wagner Instruments, model FPX 25, capacity 400 × 0.5 ozf, graduation 10 × 0.01 kgf.) before and after TENS. With the same assessor in the whole study, the assessment of the intra-rater reliability using intraclass correlation coefficients (ICC) was carried out during the familiarization session. An area of pain was located by palpation technique and marked with a non-permanent pen. The algometer was gradually pressed on the marking area until the participants gave a verbal signal to stop when the pressure reached the first perceived pain. The PPT was measured twice with a 20-second interval between assessments.
3.2.3. Heart Rate Variability
Heart rate variability (HRV) was analyzed from the ECG record using an electronic data acquisition system (PowerLab 4/30 with LabChart Pro 8 software ADInstruments). The time domain included the root mean square of successive differences (RMSSD) between normal heartbeats and the standard deviation of the interbeat interval of normal sinus beats (SDNN); however, the frequency domain included low-frequency (LF), high-frequency (HF), and LF/HF ratio parameter (LF: 0.04 - 0.15 Hz; HF: 0.15 - 0.40 Hz). Very low frequency was rarely demonstrated in all records.
3.3. Statistical Analysis
SPSS Statistics for Windows software (version 26, IBM Corp., Armonk, N.Y., USA) was used for all analyses (License: Mahidol University). GraphPad software (version 9) was used for the plot (Machine ID: D842676400D). Descriptive data are presented as frequencies for categorical variables and mean with standard deviation. The difference between before and after treatment was analyzed using the dependent t-test. Repeated one-way analysis of variance (ANOVA) was computed to identify the effect of 10 time-point values of HRV during TENS treatment. The relationship between HRV outcomes and PPT was demonstrated by scatter plots. Pearson correlation was used for testing a correlation between HRV and PPT. Statistical significance was considered at p-value < 0.05.
4. Results
4.1. Participant Characteristic
The general characteristics of 25 participants with lower limb pain are shown in Table 1. Youth participant athletes included 20 male and 5 female subjects aged between 15 to 18 years. Based on the pain rating scale (0 - 10), 10 participants had mild pain (1 - 3), and 15 participants had moderate pain (4 - 7). All 25 participants are student-athletes at the college level. They included 11 futsal players, 7 rugby players, and 5 basketball players, and the other 2 were 1 handball player and 1 taekwondo athlete. Sixteen participants showed moderated pain based on NRS scores; however, nine had mild pain scores. Among the subjects, 18 participants had tendon sprain on the patella, peroneus longus, extensor hallucis longus, abductor hallucis, extensor digitorum longus, or medial collateral ligament of the knee; nevertheless, seven of them had muscle strain on the quadriceps.
| Participation | Mean ± SD |
|---|---|
| No. (Male/ Female) | 20.5 |
| Age (y) | 16.40 ± 1.20 |
| Body mass index (kg/m2) | 20.90 ± 1.70 |
| Numeric rating scale (0 - 10) | |
| Mild pain (1 - 3) | 2.20 ± 0.63 |
| Moderate pain (4 - 7) | 4.86 ± 1.01 |
| Regular training duration (h/w) | 22.60 ± 3.80 |
4.2. Pressure Pain Thresholds and Heart Rate Variability Before and After TENS
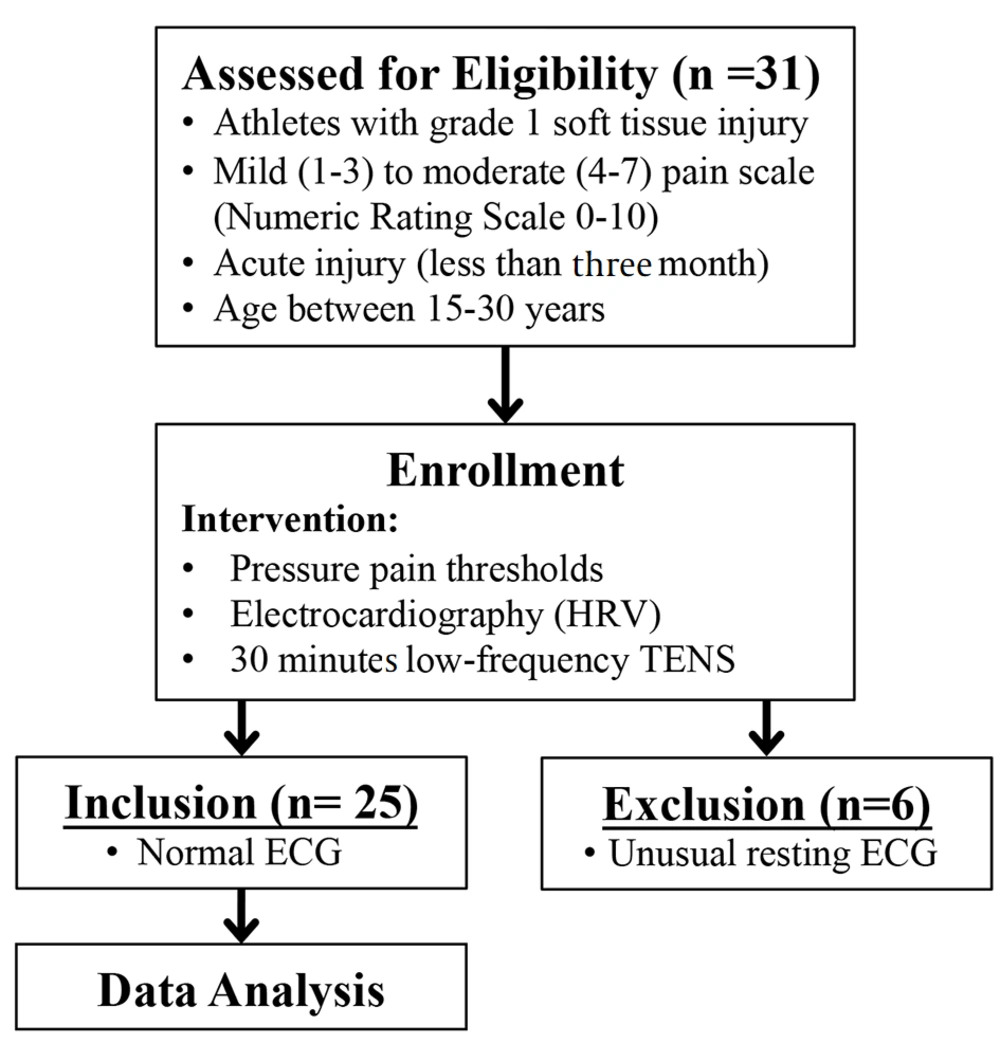
The values of PPT before and after TENS intervention are shown in Figure 2. Transcutaneous electrical nerve stimulation could significantly increase the PPT immediately after a 30-minute intervention. The baseline and post-treatment PPT values were 2.67 ± 0.79 and 2.92 ± 0.90 (P = 0.011), respectively. The data showed that 30 minutes of low-frequency electrical stimulation could immediately reduce musculoskeletal pain. However, not all participants showed an increase in the PPT. Thirteen from 25 participants (52%) demonstrated more than a 10% increase in the PPT; nonetheless, nine participants found lower than 10% effectiveness, and the PPT decreased more than 10% in three participants after the intervention. All three participants who had lower PPT after TENS treatment as compared to before treatment presented with a high rank of moderate pain score (6 and 7) from the start; nevertheless, others had a low to middle rank of moderate pain score (4 and 5).
In addition, half of the TENS responders had muscle strain injuries, and all non-responders had tendon and ligament sprain. Changes in the PPT were returned to the baseline 24 hours after the treatment (2.72 ± 0.89, P = 0.573). Heart rate variability was also compared before and after TENS treatment for 5 minutes duration each. Time-domain HRV was not significantly different between before and after treatment (SDNN, P = 0.345; RMSSD, P = 0.698). On the other hand, the frequency domain of the LF/HF ratio was significantly increased after treatment (before = 1.454 ± 0.739; after = 1.922 ± 1.378; P = 0.035); however, LF and HF were not changed (LF: P = 0.127; HF: P = 0.062). Similar to the PPT, the LF/HF ratio was returned to the baseline 24 hours after the treatment (24 hours = 1.48 ± 0.95). The HRV suggests a potential increase in sympathetic dominance after the TENS intervention.
Pressure pain thresholds (PPT) and heart rate variability (HRV) before and after 30-minute low-frequency transcutaneous electrical nerve stimulation (TENS); box plots representative of the PPT (A) and Low frequency-high frequency ratio of HRV (LF/HF) (B) of participants before and after TENS intervention. The data were obtained from 25 participants. * P < 0.05 compared to before treatment
4.3. Heart Rate Variability During TENS Intervention
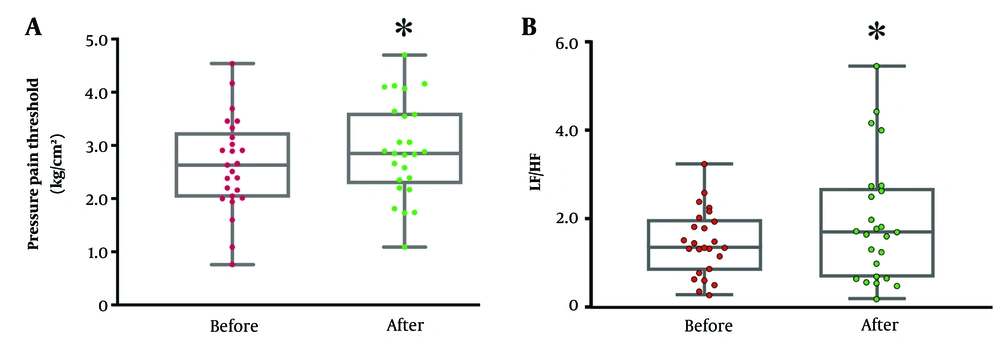
To evaluate the possible mechanism of whether electrical pain treatment triggers autonomic nervous change, the HRV was observed during TENS treatment. Time and frequency domains were calculated from 3-minute duration throughout 30 minutes of TENS treatment (Figure 3). The results showed that low-frequency electrical stimulation significantly decreased both SDNN and RMSSD over time as compared to the first 3 minutes of intervention. The effect returned to the baseline after 20 minutes of the intervention. On the other hand, there was no change in the frequency domain of HRV. The findings suggested a possible increase in sympathetic activation during intervention; however, the response was limited to around 20 minutes.
Changes in heart rate variability (HRV) during low-frequency transcutaneous electrical nerve stimulation (TENS); significant decrease in standard deviation of NN intervals (SDNN) (A), and root mean square of successive RR interval differences (RMSSD) (B), time domain HRV from baseline during the intervention; no effect on low-frequency (LF) (C), high-frequency (HF) (D), and LF/HF (E) of frequency-domain HRV during TENS intervention. The data were obtained from 25 participants. * P < 0.05 significantly compared to the baseline
4.4. Correlation Between PPT and Autonomic Outcome Variables
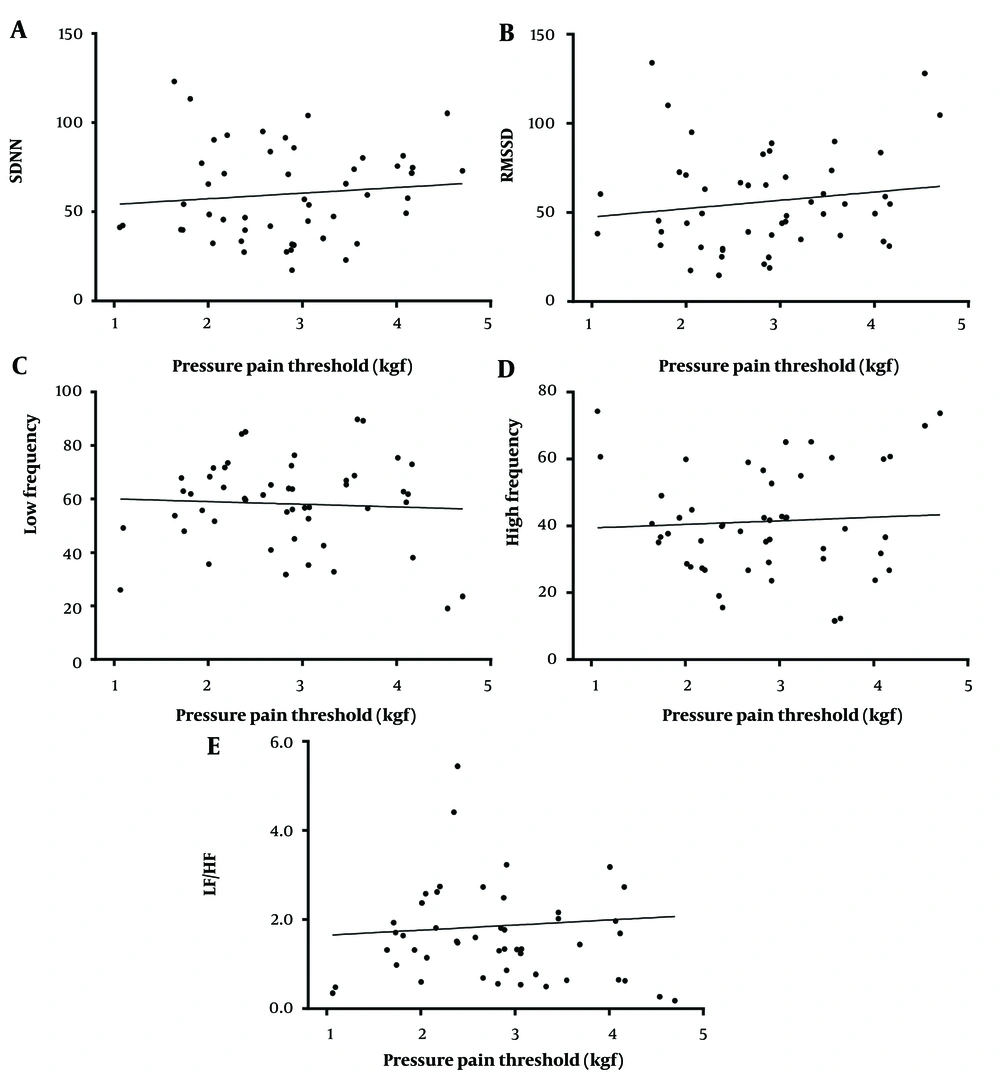
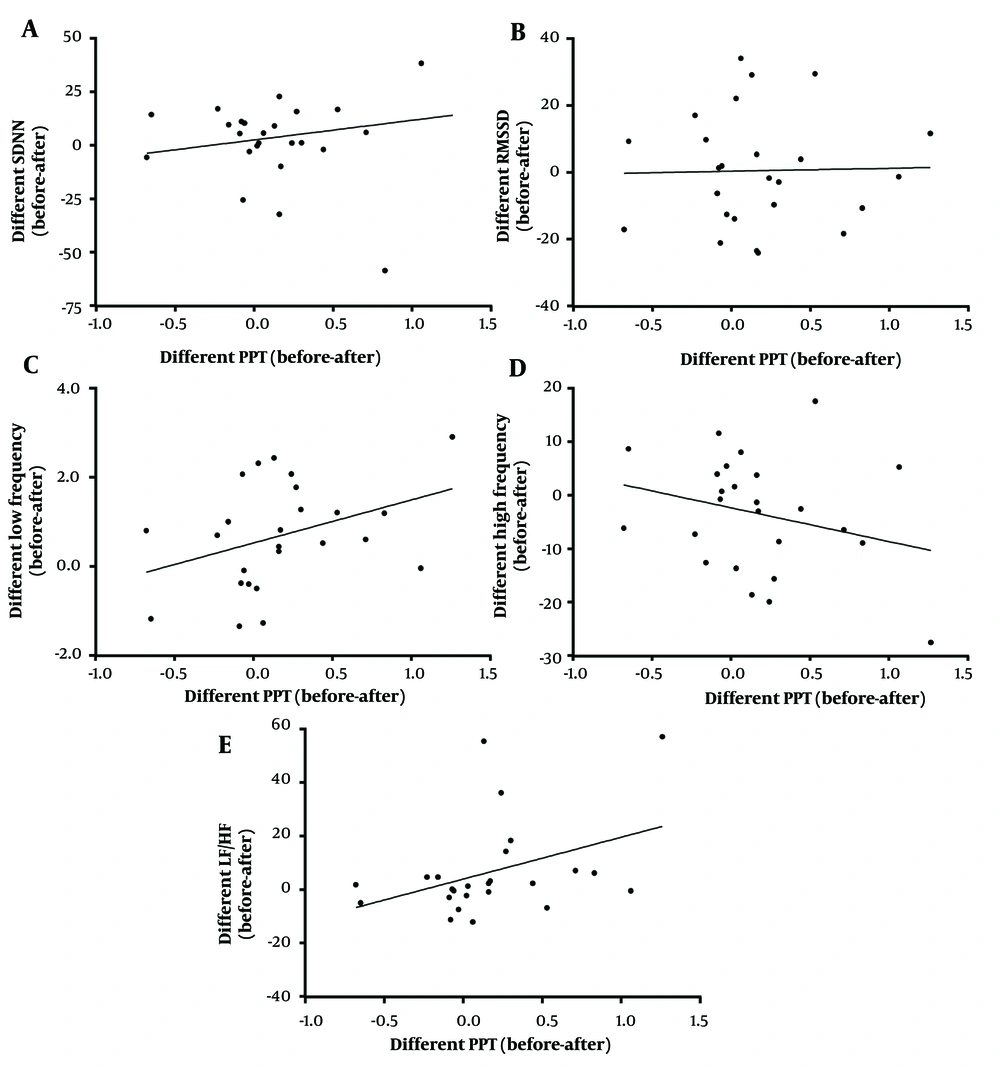
To indicate the possible relationship between pain level and autonomic activity, linear regression between the PPT (x-axis) and HRV (y-axis) were analyzed (Figures 4 and 5). Figure 4 illustrates the scatter plots of actual PPT and HRV before and after TENS treatment; however, Figure 5 depicts the mean difference between before and after treatment. For the actual value plot (Figure 4), SDNN and RMSSD had a positively negligible correlation with the PPT value (r2 = 0.0114, P = 0.46; r2 = 0.0214, P = 0.31, respectively). Low frequency exhibited a negatively weak correlation with the PPT value (r2 = - 0.0028, P = 0.71); nevertheless, HF and LF/HF had a positively negligible correlation with the PPT value (r2 = 0.0036, P = 0.68; r2 = 0.0041, P = 0.66, respectively). The correlation seems to be better when differences between before and after treatment of both HRV and PPT were analyzed (Figure 5). The difference between SDNN and RMSSD exhibited a positively negligible correlation with a difference in the PPT (r2 = 0.0372, P = 0.355; r2 = 0.0005, P = 0.911, respectively). The difference between before and after treatment of frequency-domain LF and LF/HF had a positively weak correlation with difference in the PPT (r2 = 0.1389, P = 0.066; r2 = 0.158, P = 0.049, respectively); nevertheless, difference of HF showed a negatively weak correlation with difference of PPT (r2 = 0.0712, P = 0.197).
Pressure pain thresholds (PPT)-heart rate variabilities (HRV) relationship; correlation between Actual Values of PPT and Actual Values of HRV from Before and After Intervention, Including Standard Deviation of NN intervals (SDNN) (A), root mean square of successive RR interval differences (RMSSD) (B), low-frequency domain HRV (C), high-frequency domain HRV (D), and LF/HF ratio of HRV (E) plotted based on linear regression analysis. The data were obtained from 25 participants (25 points before and 25 points after the intervention).
Correlation between the change in pressure pain thresholds (PPT) and the change in heart rate variabilities (HRV) from before to after the intervention; different values of before and after treatment of PPT and HRVs, including standard deviation of NN intervals (SDNN) (A), mean square of successive RR interval differences (RMSSD) (B), low-frequency domain HRV (C), high-frequency domain HRV (D), and LF/HF ratio of HRV (E) plotted based on linear regression analysis. The data were obtained from 25 participants.
5. Discussion
The present study aimed to evaluate the relationship between the PPT and HRV in athletes with musculoskeletal injury before and after pain relief TENS intervention. The results obtained from 25 participants demonstrated that 30 minutes of low-frequency TENS intervention significantly reduced pain; however, there was only an increase in frequency-domain LF/HF toward a decreased vagal activation. Significant decreases in both SDNN and RMSSD during the TENS procedure also implied less parasympathetic stimulation. However, there was no correlation between the actual value of PPT and the actual value of HRV in all parameters. The correlation was observed to be better when differences between before and after treatment of both HRV and PPT were determined. The obtained data suggested that the PPT difference had a higher correlation with the frequency-domain HRV than the time-domain HRV. Therefore, the change in frequency-domain HRV might be used as a signal to indicate the pain relief mechanism.
Transcutaneous electrical nerve stimulation has been used for the pain relief of various causes. High-frequency TENS has been applied and shown high effectiveness in many pain conditions, such as arthritis and neuropathic pain. Low-frequency TENS in pain relief has also been investigated for the treatment of musculoskeletal and neuropathic pain (14). Although they both stimulate sensory pathways, the pain relief mechanisms are somehow different (15). Although high-frequency TENS activates endogenous inhibitory mechanisms from the afferent fibers, low-frequency TENS activates descending inhibitory pathways involving the periaqueductal gray and rostral ventromedial medulla (PAG-RVM) pathway activating opioid, gamma-aminobutyric acid (GABA), serotonin, and muscarinic receptors to reduce dorsal horn neuron activity. The present results demonstrated the effectiveness of low-frequency TENS in reducing musculoskeletal pain. However, the relief activity was temporally less than 24 hours. Since the half-life of opioids was in the minute range (16, 17), the effect of low-frequency TENS might last for a few hours. Repeated treatment within a day was then suggested.
Similar to other previous reports, low-frequency TENS did not relieve pain in all participants within a single dose of treatment (18). Various factors might interfere with the effect, such as age, gender, initial pain level, physical fitness, stress level, and experience of pain. There was no significant difference in age, baseline PPT level, and fitness level between TENS-sensitive and desensitized groups in the present study (data not shown); nevertheless, the number of female subjects in the present study was not enough for the conclusion. Stress level and pain experience were not graded in the test.
The effect of low-frequency TENS intervention potentially decreases vagal tone to the heart. Decreases in SDNN and RMSSD during TENS treatment and an increase in LF/HF after the treatment implied that low-frequency TENS reduced the parasympathetic tone; nonetheless, sympathetic could then be dominant (19). This finding provided additional confirmation that low-frequency TENS activates the descending inhibitory pathway of nociception via sympathetic stimulation (20). Decreased HRV during TENS intervention was continued for the first 20 minutes before returning to the baseline. Whether this finding indicates the optimal stimulation of pain via descending inhibitory pathway. Previous studies demonstrated that a decrease in visual analog scores during low-frequency TENS treatment was quite constant in 20, 40, and 60 minutes of intervention (21). Based on HRV results and previous supports, 30 minutes of low-frequency TENS treatment could be enough to optimize the pain inhibitory signal.
Changes in frequency-domain HRV might reflect the pain relief. A significant relation between different PPTs and different LF/HF ratios revealed a potential to use HRV as one of the pain relief indicators. Numerous previous experiments demonstrated that HRV was well correlated with pain level (22). However, most studies were performed by inducing pain in a healthy population (22). In contrast to the present study, a systemic review by Koenig et al. in 2014 concluded that pain induction mostly increased sympathetic-baroreflex activity by either increased LF or decreased HF (23). Sympathetic arousal has been believed to be a component of pain in initiating a fight or flight response. Recent findings suggested that the cardiac ANS links in functional connection with dorsal anterior cingulate cortex and periaqueductal gray (PAG). Therefore, the activation of PAG by low-frequency TENS could possibly modulate cardiac autonomic controls. However, the role of PAG activation on sympathovagal balance is still under investigation. A study demonstrated extensive functional connectivity of both dorsolateral and ventrolateral regions of PAG in patients with posttraumatic stress disorder who have hyperarousal and active fight or flight defensive responses (24). This finding supports the possibility that PAG activation might enhance sympathetic activation as a response process.
Some limitations need to be considered in the study. Firstly, some participants did not respond to the pain relief effect of the TENS protocol used in the present study. There was no exact answer to the question. Both response and non-response groups had quite similar distribution of pain rating scale. Since all non-response participants had tendon sprain, it might be one factor affecting the TENS’s result. Secondly, the use of HRV to indicate autonomic activation was just indirect speculation. Further studies using direct measurement of neural activation are needed for confirmation. Thirdly, the present study aimed to observe the relief effect of TENS only at the first time of treatment; however, repeat treatment is practically recommended.
The present study demonstrated that a single dose of low-frequency TENS transiently relieved musculoskeletal pain. A single dose of low-frequency TENS stimulated cardiac autonomic response toward sympathetic dominance. The LF/HF domain of HRV might be the alternative parameter indicating the pain signaling activation but might not reflect the pain degree. As a result, the results suggested that all HRV parameters consisted of interindividual variation. Therefore, HRV might be suitable for the follow-up treatment but not for the rating.