1. Background
Cardiovascular diseases (CVDs) are the leading cause of death among women, particularly elderly women undergoing menopause. Reports indicate that CVDs have numerous pathological causes; however, during menopause, the dysfunction of sex hormones and subsequent metabolic disorders are significant contributors to CVDs and increased mortality in postmenopausal women with metabolic disorders (1, 2). Researchers suggest that menopause is associated with disturbances in lipid metabolism, leading to increased cholesterol and low-density lipoprotein (LDL) levels. This condition predisposes the aortic artery to blockage, which is associated with vasomotor system disorders, elevated systolic blood pressure, and reduced vascular elasticity (3).
It is believed that menopause, combined with metabolic diseases such as diabetes, results in increased levels of reactive oxygen species (ROS) and inflammatory factors such as lipocalin, endothelins, and interleukin 1-beta. This situation is accompanied by decreased levels of angiogenic markers such as vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS), as well as disruptions in angiotensin-1 and -2 (Ang-1/2) (4). Additionally, non-coding RNAs like micro-RNA (miR)-126 and miR-210 have been shown to influence angiogenesis factors. Among these, miR-126 positively affects the phosphatidylinositol 3-kinase (PI3K)/VEGF pathway, enhancing protein kinase (AKT) activity and improving angiogenesis (5). Meanwhile, miR-210 is expressed under hypoxic conditions and increases mitochondrial and cell nucleus permeability, leading to activation of the AMP-activated protein kinase (AMPK) pathway, redox regulation, and improvement of the PI3K/VEGF pathway, thereby contributing to angiogenesis (6).
Given the importance of non-invasive and cost-effective solutions for the prevention and treatment of heart diseases, researchers have recommended attention to lifestyle and regular exercise training (ET). Exercise training has been shown to improve lipid profiles and glycemic indices (7), increase mitochondrial biogenesis, and adjust cellular redox (8) in diabetic conditions. Studies have also demonstrated that regular and long-term training reduces oxidative stress and enhances biogenesis and mitochondrial function in the heart tissue of aged rats (9). Regarding the effect of ET on miRs involved in cardiovascular disease, researchers have found that high-intensity interval training (HIIT) and moderate-intensity endurance training (MIET) increase miR-126 levels, which leads to higher VEGF levels and PIK3R2, as well as a reduction in vascular cell adhesion molecule-1 (VCAM-1) and inflammatory factors in the heart tissue of type 2 diabetic rats. The type of ET influences these effects; long-term endurance ET has a more favorable impact than resistance ET (10). Another study showed that endurance swimming training increased miR-1 and sodium/calcium exchanger-1 (NCX-1), with miR-1 exhibiting anti-angiogenic effects. Swimming training, by increasing miR-1, disrupted its expression in an animal model of myocardial infarction (11). Therefore, it appears that ET has varying effects on oxidative stress, inflammation, and sometimes angiogenesis under certain conditions, such as heart attacks, and its molecular and cellular mechanisms are not yet fully understood in this context (12).
In addition to the positive effects of exercise training, the use of natural antioxidant-rich foods in the treatment of cardiovascular diseases (CVDs) and traditional medicine has always been of interest. Royal jelly, due to its various flavonoids, glucosides, saturated fatty acids, and vitamins, may have antioxidant, anti-inflammatory, and anti-apoptotic effects, and is effective in treating metabolic diseases such as diabetes (13, 14). For instance, researchers demonstrated that RJ consumption significantly reduced glycated hemoglobin (HbA1c), improved lipid profiles, and enhanced apolipoprotein A-1 (Apo-A-1) levels in diabetic patients (14). Additionally, a study showed that a daily intake of 650 mg of RJ for four weeks improved hyperglycemia indices and liver enzymes in diabetic patients (15). Furthermore, researchers reported that supplementing with 50 and 100 mg/kg of RJ for eight weeks decreased malondialdehyde (MDA), increased catalase (CAT) and glutathione (GSH), and reduced apoptotic and inflammatory markers in the liver tissue of rats, with no significant difference observed between the different doses (16).
Regarding the combined effects of exercise and antioxidant consumption in metabolic diseases and menopause, researchers found that ET and estrogen supplementation led to an increase in antioxidant enzymes in ovariectomized rats (17). Additionally, ET and RJ consumption increased superoxide dismutase (SOD) and glutathione peroxidase (GPx) levels in the heart tissue of diabetic ovariectomized rats, with RJ showing a more pronounced effect on GPx than ET (18). Another study showed that both aerobic training and RJ consumption, either alone or in combination, improved SOD and GPx levels and reduced MDA in the liver of obese rats; however, the combined effect of these interventions was less effective than each intervention alone (19). Studies indicate limited information about angiogenic factors in vessels close to the heart and their response to ET and antioxidant consumption. Although the beneficial effects of training and RJ consumption on metabolic markers have been reported, further investigation into the mechanisms of angiogenesis related to miR-210 and miR-126 in the aortic artery could provide more comprehensive insights.
2. Objectives
The aim of this study was to investigate the angiogenic effects of aerobic training (AT) and RJ consumption, with a focus on the roles of miR-126 and miR-210 in the aortic artery of ovariectomized diabetic (OVXD) rats.
3. Methods
Considering the nature of this research, which was experimental, 40 female Sprague-Dawley rats, aged 14 ± 2 weeks and weighing 230 ± 15 grams, were prepared and transported to the ET physiology laboratory. To adapt to the environment, the rats were acclimated in the laboratory for one week. During the research period, the rats were maintained under standard conditions, which included controlled temperature, light, humidity, a light-dark cycle, free access to water, and specialized rat food. The ethical principles for working with laboratory animals were strictly followed according to the Hellenic Treaty. This study was approved under the ethical approval code IR.IAU.SDJ.REC.1400.042.
It is worth noting that the special food for the rats was prepared by Daneh Pars Company (Iran). This diet was designed to contain 58% carbohydrates, 13% fat, and 28% protein.
3.1. Ovariectomy Method and Induction of Diabetes
After the adaptation period, 34 rats underwent surgery to remove the ovaries. For this purpose, the rats were first anesthetized using ketamine (50 mg/mL) and xylazine (20 mg/mL). After ensuring complete anesthesia and shaving the abdominal hair, a 3 cm incision was made along the midline of the abdomen by cutting through the muscle layers and peritoneum. Following visualization of the ovaries and uterus, the fallopian tubes were first ligated with absorbable surgical thread (size 3 - 0), and then the ovaries were excised using a surgical blade. The incision was closed with a single-layer vicryl suture (size 3 - 0), and to prevent infection and support recovery, the animals were kept in a fully standard environment and recovery room in individual boxes. An OTC solution was also used to prevent infection. The rats were then maintained in the laboratory for 3 months to allow the effects of estrogen deficiency on metabolism to become apparent. Subsequently, diabetes was induced in the operated rats using a single intraperitoneal injection of 40 mg/kg streptozotocin, manufactured by Sigma-Aldrich Company in the United States. Four days after the STZ injection, blood glucose levels were tested by creating a small wound in the tail of the animals and using a glucometer made in Germany. Rats with blood glucose levels above 250 mg/dL were classified as diabetic. Two rats were removed from the study due to blood glucose levels below 100 mg/dL. (20) It is worth noting that 2 rats were removed from the study due to blood glucose levels below 100 mg/dL, and an additional 2 rats were removed due to individual reactions to streptozotocin and an excessive increase in blood glucose. Consequently, 30 ovariectomized diabetic rats were divided into 5 groups based on blood glucose levels: (1) OVXD, (2) sham (Sh), which received RJ solvent, (3) RJ, (4) AT, and (5) AT + RJ. Additionally, to investigate the effects of ovariectomy and diabetes induction, 6 healthy rats were selected as the healthy control group (HC).
3.2. Aerobic Training Protocol
To perform aerobic training, the rats were initially introduced to the treadmill for one week. During this acclimation period, they were placed on the treadmill at a speed of 8 m/min for 5 - 10 minutes. Following this, the maximum running speed was assessed. The procedure involved a 5-minute warm-up at a speed of 5 m/min, followed by an incremental increase of 1 m/min every three minutes until the rats reached exhaustion (defined as the inability to run due to fatigue and hitting the end of the treadmill three times in succession).
In this study, aerobic training was conducted such that, during the first week, the rats ran on the treadmill at a speed of 20 m/min, which was 55% of the maximum running speed. In the subsequent weeks, 10 minutes were added to the training time each week until, by the fourth week, the rats reached the maximum running speed initially measured, with the training duration extending to one hour. According to the protocol of Souza et al., this intensity was equivalent to 55 - 75% of the maximum speed in the eighth week. The training regimen in this study was maintained for eight weeks, with five sessions per week. Additionally, a 5-minute cooldown period was included, during which the treadmill speed was gradually reduced. (21)
3.3. Royal Jelly Consumption
Royal jelly supplementation was administered daily. For this purpose, 300 mg of fresh RJ, prepared from the Agricultural Jihad of Marvdasht city, was dissolved in 3.6 mL of injectable sodium chloride (9%). Then, 0.3 mL of this solution was injected intraperitoneally into each rat. It is worth noting that this injection volume was equivalent to 100 mg/kg (22).
3.4. Sampling
Forty-eight hours after the last training session, the rats were anesthetized with a solution of 50 mg/mL ketamine and 20 mg/mL xylazine after a 12-hour fast. Once unconsciousness and absence of pain were confirmed, the thoracic cavity of the animals was opened. The aortic artery tissue was carefully extracted after discarding other tissues and cutting the arteries entering and leaving the heart. The aortic artery tissue was then immediately immersed in a nitrogen tank and kept at -80°C until the variables were measured.
3.5. Methods of Measuring Variables
To measure VEGF, eNOS, miR-210, and miR-126, the following steps were performed. First, aortic artery tissue was homogenized in phosphate-buffered saline. Next, 50 mg of tissue was separated for RNA extraction, which was carried out according to the manufacturer's protocol (Qiagen, Germany). The quality of RNA was assessed using agarose gel electrophoresis and by measuring optical absorption at a wavelength of 260 nm with a Sigma Pico Drop device (USA). The formula (C (μg/μL) = A260 × ε × d/1000) was used for quantification. Once the desired concentration was confirmed, the samples were prepared for subsequent steps.
Following RNA extraction, cDNA synthesis was performed using the manufacturer's protocol in the Fermentas kit (K1621), along with the designed primers (Table 1) based on guidelines from the VEGF, eNOS, miR-210, and miR-126 genes on the PUBMED website. Reverse transcription was conducted to amplify these genes. The efficiency and specificity of the primers were evaluated using software available on the NCBI website. To measure gene expression levels, internal control genes TBP and U6 were used. After completing the qReal Time PCR process and ensuring that samples reached the expression threshold (Cycle Threshold), the 2-ΔΔCT formula was applied to quantify the ratio of the target gene to the reference gene.
| Genes | Primer Sequences; (5’-3’) | Sizes (bp) |
|---|---|---|
| TBP | 147 | |
| Forward | GCGGGGTCATGAAATCCAGT | |
| Reverse | AGTGATGTGGGGACAAAACGA | |
| VEGF | 183 | |
| Forward | ACTTGAGTTGGGAGGAGGATGTC | |
| Reverse | GGATGGGTTTGTCGTGTTTCTGG | |
| eNOS | 60 | |
| Forward | TGACCCTCACCGATACAACA | |
| Reverse | CGGGTGTCTAGATCCATGC | |
| miR-126 | - | |
| Forward | AGCGATGATGCACTGTCAGAA | |
| Reverse | AACGGAACTCCAGAAGACCAG | |
| miR-210 | - | |
| Forward | CGCCTGTGCGTGTGACAGCG | |
| Reverse | GTGCAGGGTCCGAGGT | |
| U6 | - | |
| Forward | CTCGCTTCGGCAGCACA | |
| Reverse | AACGCTTCACGAATTTGCGT |
Sequence of Primers Used in the Research
3.6. Statistical Analysis Tests
To analyze the data in this study, the Shapiro-Wilk test was first employed to assess the normality of the data distribution. Based on the distribution results, one-way analysis of variance (ANOVA) was used to evaluate differences between groups, with Tukey's post-hoc test applied to pinpoint the specific group differences. Additionally, two-way ANOVA with Bonferroni's post-hoc tests was conducted to explore the main effects of exercise and supplementation, as well as their interaction and effect size. Data analysis was performed using GraphPad Prism 8.3.3 software, with significance set at (P ≤ 0.05).
4. Results
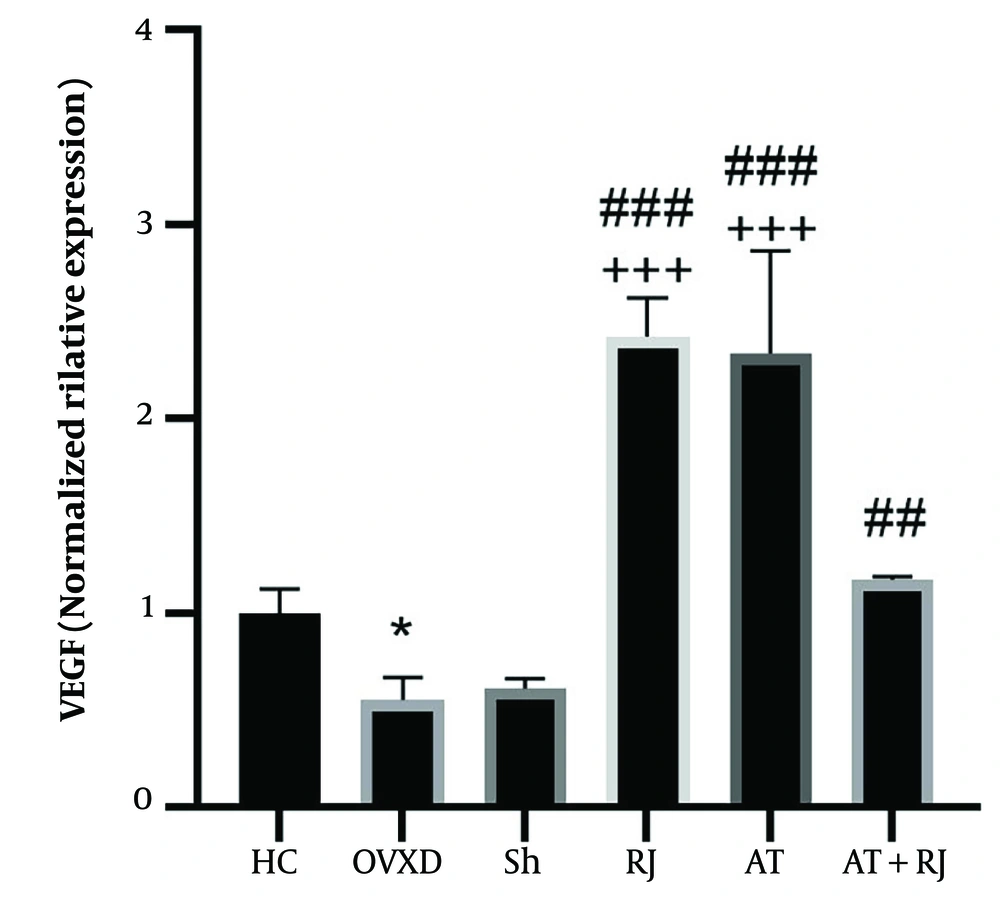
The results of the one-way analysis of variance test indicated significant differences in the levels of VEGF (P = 0.001), eNOS (P = 0.001), miR-126 (P = 0.001), and miR-210 (P = 0.001) among the research groups. Tukey's post-hoc test revealed that VEGF levels in the OVXD group were lower than those in the HC group (P = 0.03). However, VEGF levels were significantly higher in the RJ (P = 0.001), AT (P = 0.001), and AT + RJ (P = 0.0014) groups compared to the OVXD group, as well as in the RJ (P = 0.001), AT (P = 0.001), and AT + RJ (P = 0.004) groups compared to the Sh group. No significant difference was observed between the RJ and AT groups (P = 0.98), but VEGF levels were significantly higher in the RJ (P = 0.001) and AT (P = 0.001) groups compared to the AT + RJ group.
Two-way analysis of variance demonstrated that both supplementation (F = 9.01, P = 0.007, effect size 0.31) and training (F = 5.13, P = 0.03, effect size 0.20) significantly affected VEGF levels. The interaction between training and supplementation on VEGF levels was also significant (F = 165.27, P = 0.001, effect size 0.31) (Figure 1).
Vascular endothelial growth factor (VEGF) gene expression levels in the aortic artery tissue of rats in research groups. * (P ≤ 0.05) significant decrease compare to HC group. ## (P ≤ 0.01) and ### (P ≤ 0.001) significant increase compared to ovariectomized diabetic (OVXD) group. +++ (P ≤ 0.001) significant increase compared to AT + RJ group
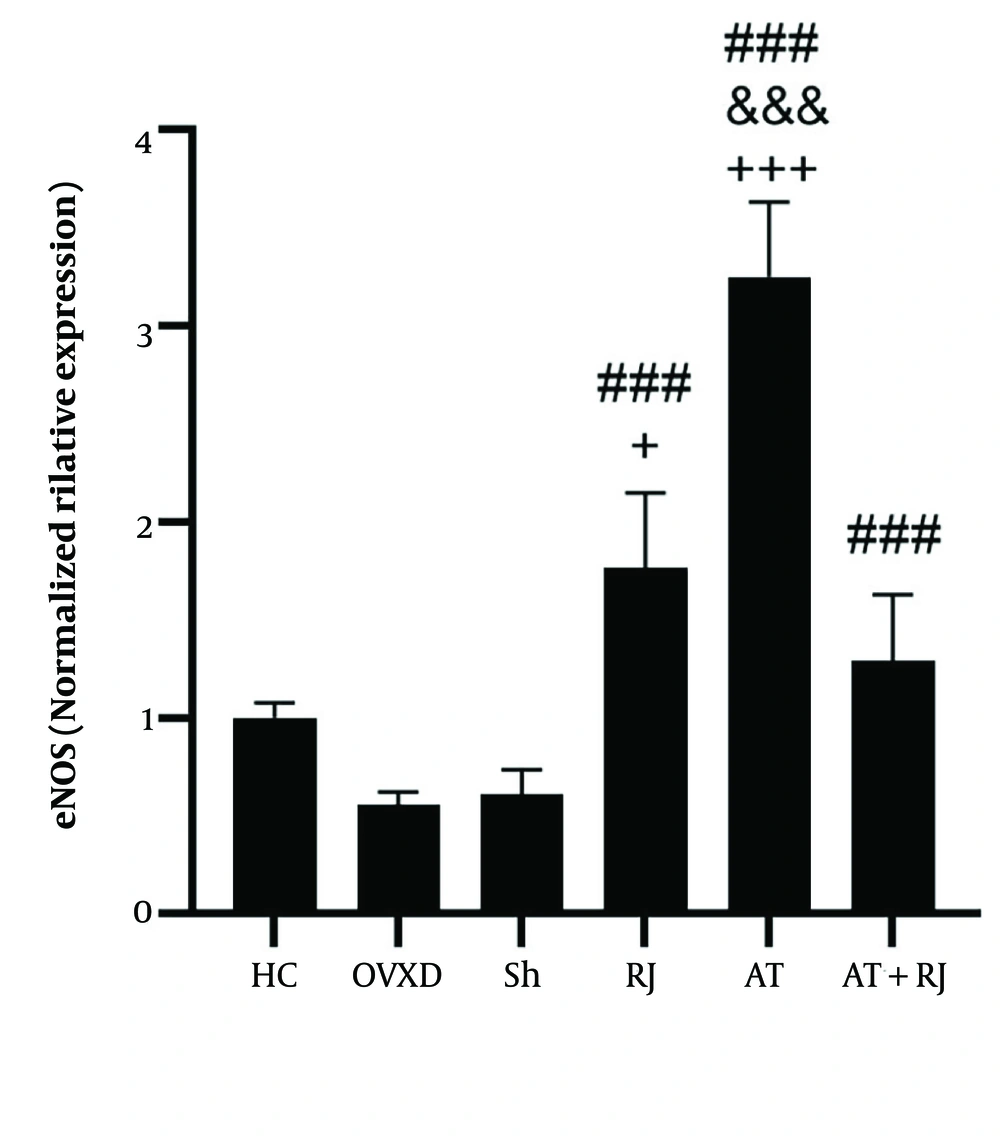
For eNOS levels, no significant difference was found between the HC and OVXD groups (P = 0.07). However, eNOS levels were significantly higher in the RJ, AT, and AT + RJ groups (P = 0.001) compared to the OVXD group, and also higher in the RJ (P = 0.001), AT (P = 0.001), and AT + RJ (P = 0.0014) groups compared to the Sh group. Additionally, eNOS levels were higher in the AT group compared to the RJ group (P = 0.001), and higher in the RJ (P = 0.04) and AT (P = 0.001) groups compared to the AT + RJ group. The analysis showed that both supplementation (F = 8.30, P = 0.009, effect size 0.29) and training (F = 72.65, P = 0.001, effect size 0.78) had significant effects on increasing eNOS levels. The interaction of training and supplementation on eNOS levels was also significant (F = 147.56, P = 0.001, effect size 0.88) (Figure 2).
Endothelial nitric oxide synthase (eNOS) gene expression levels in the aortic artery tissue of rats all groups. ### (P ≤ 0.001) significant increase compared to ovariectomized diabetic (OVXD) group. &&& (P ≤ 001) significant increase compared to the RJ group. + (P ≤ 05) and +++ (P ≤ 001) significant increase compared to AT + RJ group
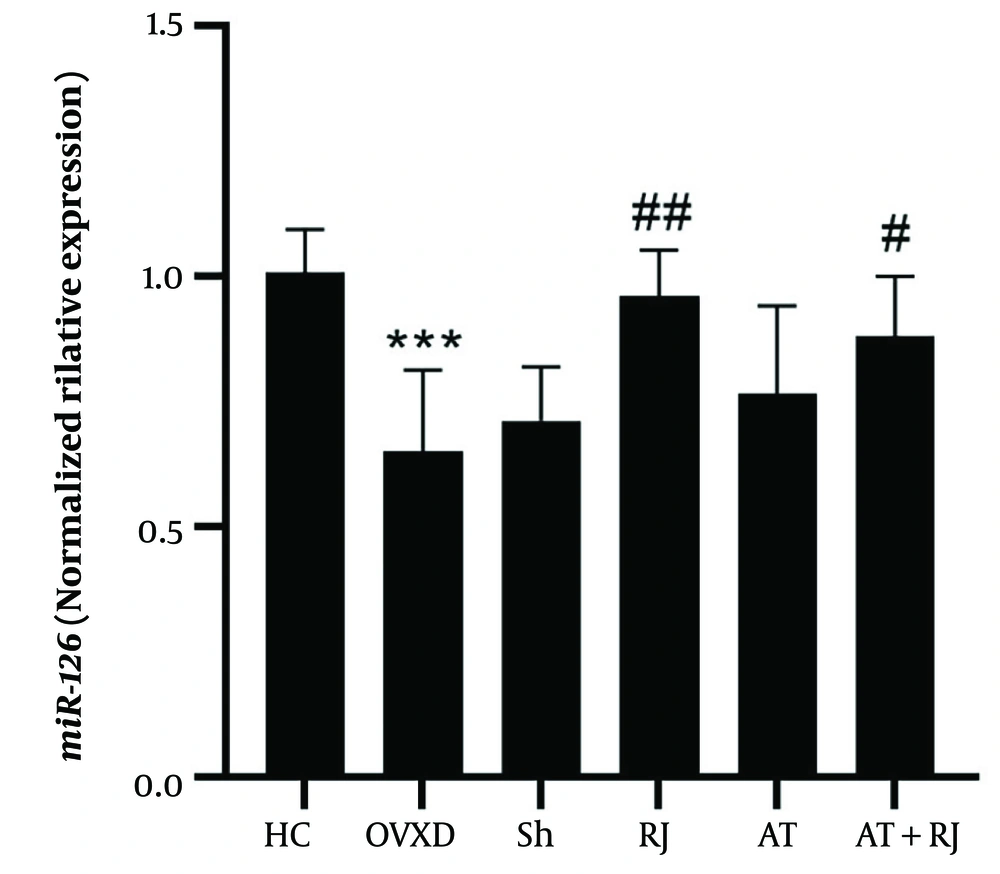
miR-126 levels in the OVXD group were significantly lower than in the HC group (P = 0.001). However, miR-126 levels were higher in the RJ (P = 0.002) and AT + RJ (P = 0.04) groups compared to the OVXD group. The analysis revealed a significant effect of supplementation on increasing miR-126 (F = 13.50, P = 0.002, effect size 0.40). Conversely, the effect of training (F = 0.08, P = 0.77, effect size 0.004) and the interaction between training and supplementation on miR-126 were not significant (F = 2.86, P = 0.10, effect size 0.12) (Figure 3).
miR-210 levels in the OVXD group were significantly lower than those in the HC group (P = 0.001). In contrast, miR-210 levels were significantly higher in the RJ (P = 0.001), AT (P = 0.01), and AT + RJ (P = 0.001) groups compared to the OVXD group. Additionally, the AT + RJ group had significantly higher miR-210 levels than the RJ (P = 0.001) and AT (P = 0.001) groups. The analysis indicated that both supplementation (F = 69.06, P = 0.001, effect size 0.77) and training (F = 45.64, P = 0.001, effect size 0.69) had significant effects on increasing miR-210 levels. However, the interactive effect of training and supplementation on miR-210 was not significant (F = 2.29, P = 0.14, effect size 0.10) (Figure 4).
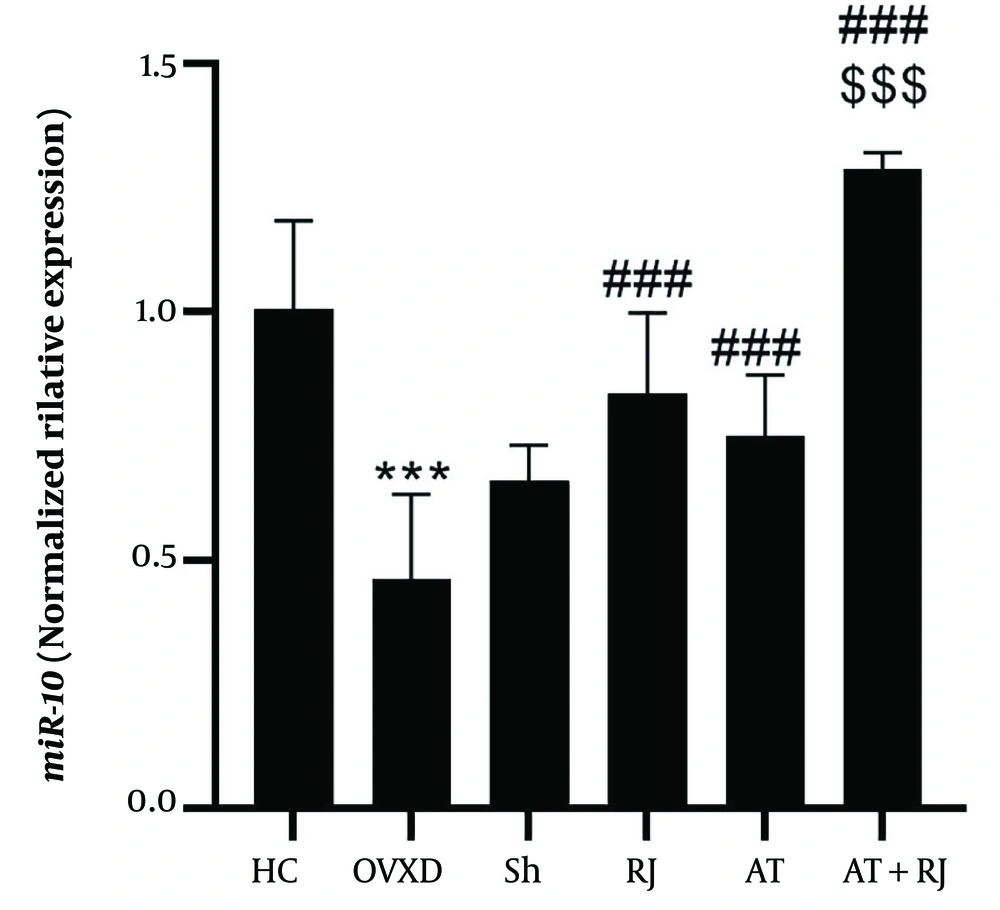
miR-10 gene expression levels in the aortic artery tissue of rats in research groups. ***(P = 0.001) significant decrease compared to HC group. ### (P = 0.001) significant increase compared to ovariectomized diabetic (OVXD) group. $$$ (P = 0.001) significant increase compared to RJ and AT groups
5. Discussion
The results of the present study indicated that VEGF, miR-126, and miR-210 levels were significantly lower in the OVXD group compared to the HC group. However, aerobic training (AT) significantly increased VEGF, eNOS, and miR-210 levels. Researchers have suggested that diabetes elevates free radicals by disrupting energy substrate metabolism, leading to increased inflammation and apoptosis. This disruption in cellular redox can inhibit the phosphorylation of proteins essential for angiogenesis (23). In type 2 diabetes, researchers observed decreased ratios of p-PI3K to PI3K, p-Akt to Akt, p-eNOS to eNOS, and p-VEGFR2 to VEGFR2, along with reduced VEGF protein levels and increased levels of hemoxygenase-1 (HO-1), caspase-3, NF-κB, and TNF-α (23).
Additionally, disturbances in hormones such as estrogen due to diabetes can impact muscle anabolic processes, affecting muscle mass and increasing fat mass. Lower estrogen levels, as seen in diabetes or menopause, are also associated with cardiac atrophy, which may lead to increased right ventricle thickness, elevated blood pressure, and cardiac fibrosis (24). Specifically, miR-126, through its subunits miR-126-3p and miR-126-5p, influences vascular angiogenesis by regulating proteins such as PIK3R2, SPRED1, VCAM-1, TRAF7, HMGB1, and ALCAM. In diabetes, reduced miR-126 levels inhibit the expression of insulin substrate receptor (IRS-1), a key factor in cardiovascular disease diagnosis (10). Other studies have shown that decreased expression of miR-210 and miR-126 is linked to impaired Akt phosphorylation, ERK1/2, and VEGF, ultimately disrupting vascular angiogenesis (25).
Regarding the impact of physical activity on miR-210 and miR-126-dependent angiogenesis pathways, it has been demonstrated that eight weeks of voluntary exercise training led to increased levels of Akt, ERK1/2, miR-210, miR-126, and CD31 in rat heart tissue (25). Another study found that voluntary exercise training improved the lipid profile and increased miR-210 and miR-126 levels in the heart tissue of diabetic rats (26). Additionally, ten weeks of aerobic swimming training was shown to promote favorable differentiation of heart muscle fiber types, improve miR-16 and miR-126 levels, increase eNOS, and reduce blood pressure and apoptotic markers in rat heart tissue (27).
Thus, regular and long-term physical activities enhance lipid profiles, decrease NF-κB and TNF-α, improve PIK3R2 expression, and increase nitric oxide (NO) and p-eNOS levels. These effects contribute to elevated vascular sprouting proteins and facilitate angiogenesis through the expression of miRNAs like miR-16 and miR-126 (27). Moreover, researchers have noted that exercise training increases reactive oxygen species (ROS), activating redox pathways, which in turn boost NO levels. Elevated NO levels subsequently lead to eNOS phosphorylation and FOXO protein activation. Increased FOXO protein levels enhance the phosphorylation of VEGF and its receptor, further facilitating angiogenesis (28).
The results of the present study demonstrated that RJ consumption increased the levels of VEGF, eNOS, miR-126, and miR-210. Research indicates that RJ contributes to reducing metabolic disorders by neutralizing free radicals. Specifically, the presence of 10-hydroxy-2-decenoic acid (10H2DA) in RJ allows it to bind to free radicals, thereby reducing lipid peroxidation. Additionally, RJ consumption has been associated with increased levels of high-density lipoprotein and apolipoprotein A-1 (Apo-A1), which in turn leads to decreased levels of cholesterol, low-density lipoprotein, and triglycerides. Consequently, the observed reduction in blood pressure following RJ use can be attributed to improvements in NO, angiotensin-converting enzyme-1 (ACE-1), VEGF, VCAM, and ICAM levels (14, 29).
Moreover, RJ appears to activate AMPK, which subsequently leads to IRS-1 activation, Akt phosphorylation, increased expression of glucose transporter-4 (GLUT4), ERK1/2 phosphorylation, and enhanced VEGF expression (14). Researchers have highlighted that RJ consumption improves lipid profiles and glycemic indices, boosts both enzymatic and non-enzymatic antioxidants, and reduces oxidative damage (14). Additionally, a review study found that RJ plays a role in regulating miRNAs in metabolic diseases and is effective in cardiovascular conditions by enhancing miR-210 (30). Another study reported that selenium-rich RJ led to increased levels of MDA, TNF-α, prostaglandin F1β, PI3K, AKT phosphorylation, and apoptotic markers, while inhibiting cyclooxygenase-2 (COX-2) and VEGF in cancer cells (31).
The present study was consistent with previous research, and no studies were found to be contradictory to our findings. In this study, the levels of VEGF, eNOS, miR-126, and miR-210 in the AT + RJ group were significantly higher than in the OVXD group. However, the effects of AT and RJ separately on increasing VEGF and eNOS were more pronounced than the combined effect of AT + RJ. On the other hand, the interactive effect of AT + RJ on the increase of miR-126 was more beneficial than either intervention alone. Consistent with our findings, researchers have demonstrated that endurance training and RJ consumption resulted in increased NO, ACE-I, and VEGF in rats with L-NAME-induced hypertension (29). Additionally, aerobic training and RJ consumption led to increased SOD and GPx levels, as well as decreased MDA levels in the left ventricular tissue of OVXD rats (18). Dariushnejad et al. found that voluntary endurance training and crocin consumption together increased miR-21 and miR-12 in the heart tissue of diabetic rats (32). Another study showed that the combination of endurance training and crocin improved Akt, ERK1/2, miR-210, and miR-126, ultimately enhancing angiogenesis in the hearts of rats (25).
Conversely, using antioxidants in conjunction with exercise training has been debated. Some researchers argue that antioxidants (depending on the dosage) might neutralize ROS, leading to the inhibition of redox pathways and especially reducing NO and eNOS (33, 34). Supporting this view, our study also found that AT had a more favorable effect on increasing eNOS compared to RJ or the combined effect of RJ and AT. Given the improvements observed in markers of angiogenesis and angiomir expression following these two interventions, it may be possible to use them in clinical trials with postmenopausal women with diabetes, provided caution and ethical principles are observed.
One limitation of this study is the lack of investigation into ROS levels, which are crucial for activating biological pathways following exercise training. Future research should evaluate oxidant-antioxidant markers. Additionally, the study did not assess extracellular kinase pathways such as Akt and ERK1/2, which play roles in both AT and RJ effects. It is recommended to explore these pathways in future studies. Another limitation is the use of a single dose of antioxidants alongside exercise training. Since antioxidant dosage is critical, future studies should evaluate different doses to determine the optimal response in angiogenesis. Lastly, the study did not assess the effects of different doses of royal jelly on oxidative stress and angiogenesis. Therefore, evaluating various doses of royal jelly in conjunction with exercise training is suggested for future research.
5.1. Conclusions
It seems that while both AT and RJ supplementation, whether alone or in combination, increase markers of vascular angiogenesis, the influence of vascular angiogenesis on oxidative stress and eNOS suggests that the use of antioxidants alongside exercise training might modulate this mechanism.