1. Background
Plantar fasciitis (PF) is one of the most prevalent causes of heel pain globally and is characterized by plantar fascia thickening. At least one in ten individuals will experience PF in their lifetime (1). Despite the term "plantar fasciitis," recent histological findings reveal degenerated collagens and disoriented fibers without inflammatory cells. The condition is therefore more accurately described as fasciopathy rather than fasciitis (2, 3). The precise cause of PF is poorly understood, but it has been observed that this condition is more prevalent in sedentary populations with improper foot structure (4).
There are numerous non-surgical treatments available, such as night splints, eccentric stretching exercises, orthotics, and medications such as non-steroidal anti-inflammatory drugs non-steroidal anti-inflammatory drugs (NSAIDs) (5). These techniques provide pain relief for 80% of patients. After the failure of non-invasive and non-steroidal treatments, corticosteroid injections such as methylprednisolone are the next therapeutic option (6).
Unfortunately, a number of studies have demonstrated that corticosteroid injections relieve pain in the short term but have negative effects and complications over time. These complications include rupture of the plantar fascia, thinning of the plantar adipose pad, injury to peripheral nerves, changes in skin pigmentation, post-injection flare, infection, and muscle damage, which necessitate the development of other novel therapies (7-9). Several studies have demonstrated that the injection of platelet-rich plasma (PRP) has a positive influence on this fasciopathy (10, 11). Platelet-rich plasma is a component of blood that has been centrifuged and is composed primarily of platelets and other growth factors (12). These factors promote angiogenesis, cell differentiation, collagen synthesis, and tissue regeneration. In addition, the anti-inflammatory effects of the cytokines present in PRP help decrease discomfort (13, 14). However, there is limited solid evidence of the long-term efficacy and optimal dosage of PRP in the treatment of PF.
Several studies have compared the efficacy of corticosteroid injections versus PRP injections in treating PF. Various dosages of methylprednisolone and PRP were utilized in these investigations with different populations, follow-up periods (mainly between three weeks to three months), and outcomes.
2. Objectives
We designed this clinical trial to compare the effects of PRP and corticosteroids in the treatment of PF.
3. Methods
3.1. Population and Study Design
In this clinical trial, 30 patients with PF were assigned to participate in two groups (PRP vs. corticosteroids, 15 each) in a tertiary care hospital in Tehran, Iran, in 2022. According to previous articles and the prevalence of the disease, the sample size (considering a 5% type 1 error, 5% type 2 error, 20% attrition rate, effect size of 13.6%, 0.05 alpha, and 0.8 power) was calculated to be 15 in each study group. Randomization was implemented to ensure that each participant had an equal chance of being assigned to either the treatment or control group, thereby minimizing selection bias. A random number generator was used to create a random allocation sequence. This sequence was prepared by an independent statistician not involved in the trial. Sequentially numbered, opaque, sealed envelopes (SNOSE) containing the group assignments were prepared based on the random sequence. Each envelope was opened only after a participant was deemed eligible and had consented to participate.
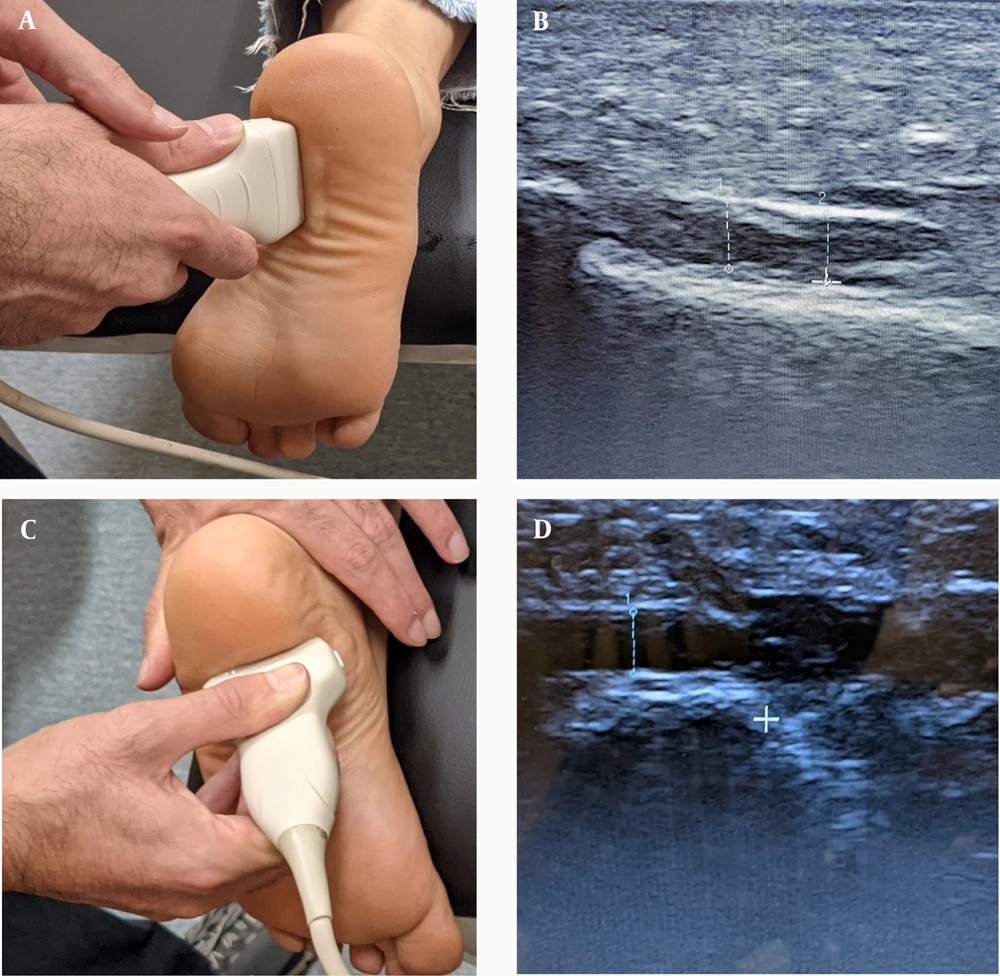
Ultrasonography confirmed the definitive diagnosis of PF in patients with chronic heel pain within the past three months. The inclusion criteria were as follows: (1) age of 18 or older; (2) presence of symptoms (such as heel pain, swelling, redness, tenderness, and stiffness) for more than three months with no response to supportive treatment; (3) ultrasound examination revealing a diseases, and coagulation disorders; (6) Infection or injury at the injection site; (7) Corticosteroid injections in the area within the last three months or NSAID use within the last week. plantar fascia thickness (PFT) of over 4 mm; (4) Numeric Pain Rating Scale (NPRS) of six or greater; (5) No alternative diagnoses in the area, such as fractures or neurological disorders. Exclusion criteria included: (1) other treatments administered to the patient during the follow-up period that deviated from the protocol (such as topical ointments); (2) not routinely participating in the exercises during the follow-up period; (3) excruciating pain equivalent to an NPRS score of 10, which interfered with the patient's function and was not alleviated by short-term NSAID use; (4) pregnancy; (5) history of any of the following conditions: Diabetes mellitus, cancer, rheumatologic Prior to intervention, primary information was obtained from the participants regarding age, gender, anthropometric indices, and duration of symptoms. Additionally, PFT was measured by a final-year sports medicine resident using a portable ultrasound device (TELEMED ultrasound MicrUs EXT-1H) (Figure 1). Several questionnaires regarding pain and foot function, including NPRS, Roles and Maudsley score (RMS), and the Foot and Ankle Disability Index (FADI), were completed by the patients.
Blinding was employed to reduce bias and ensure the integrity of the data. Participants were unaware of whether they were receiving the PRP injection or the corticosteroid injection. Both treatments were administered in a similar manner to maintain blinding. The healthcare providers administering the treatments and those assessing the outcomes were blinded to the group assignments. The injections were prepared by a separate, unblinded pharmacist who did not participate in any other aspect of the trial.
3.2. Intervention
The corticosteroid group received a one-time injection of 40 mg of methylprednisolone with 1 cc of 2% lidocaine, administered at the site of maximum tenderness. For the PRP group, the O.PRP kit (Noavaran Salamat Arjang, Tehran, Iran) was used. This kit contains two separate bags. Initially, 5 cc of 3.8% sodium citrate was injected as an anticoagulant. Then, 10 cc of blood was drawn from the patient and placed directly into the first bag. The sample was centrifuged twice to ensure maximal platelet concentration. In the first centrifugation round, after 15 minutes at 1200 rpm, the plasma and platelets were separated from the red blood cells and transferred to the second container. In the second round, the sample was centrifuged for 6 minutes at 2700 rpm (15). Finally, the precipitated platelets and a small amount of remaining plasma were mixed, and 2 cc of the mixture was injected one time, blinded, at the site of maximum tenderness (Figure 2).
3.3. Post-Intervention
After the injection, patients in both groups were instructed to perform a combination of mobilization, stretching, and strength exercises, as well as soft tissue release. They were advised to begin exercising 48 hours after the injection and to perform each exercise five days per week.
3.3.1. Mobilization Exercise
This included writing letters of the alphabet with the toes. While seated on a chair or cot with their legs dangling, patients drew letters of the alphabet using their toes and the front part of their foot. This motion was repeated three times for a total of ten sets.
3.3.2. Stretching Exercises
1. Stretching the gastrocnemius muscle by performing three sets of lunges, holding each stretch for 15 to 30 seconds.
2. Stretching the soleus muscle by performing three sets of deep lunges, holding each stretch for 15 to 30 seconds.
3. Stretching the plantar fascia by holding the front of the foot and pulling it towards the dorsum for three sets of three reps, each lasting 15 to 30 seconds.
3.3.3. Strength Exercises
This included strengthening the plantar foot muscles using a cloth or a marble. With the towel on a flat surface and without lifting the heel from the floor, patients lifted the towel from the floor with their toes, brought it towards themselves, held it for five seconds, and then released it. This exercise was performed for ten reps in three sets.
3.3.4. Soft Tissue Release
This was performed with a bottle of frozen water. While seated in a chair, patients placed the bottle under their feet and rolled it from the origin of the fascia to the front of the foot for five minutes, performing three to five sets.
3.4. Outcome Measurements
To ascertain the patient's pain level, the NPRS was used. Patients rated their pain on a scale from 0 to 10, with 0 indicating no pain and 10 indicating unbearable and excruciating pain. In this trial, the RMS and FADI scales were also used to assess patients' foot function and level of satisfaction (16-18). All evaluations and ultrasound examinations were repeated at the first and second follow-up visits (one and three months after the intervention).
3.5. Statistical Analysis
SPSS version 25 was used. The Kolmogorov-Smirnov test was used to assess the normal distribution of variables. The chi-square test, Kendall’s Tau-b, Fisher’s exact test, Mantel-Haenszel test, independent t-test, and Mann-Whitney U test were used to compare variables between study groups. A P-value of less than 0.05 was considered significant.
4. Results
4.1. Baseline Characteristics
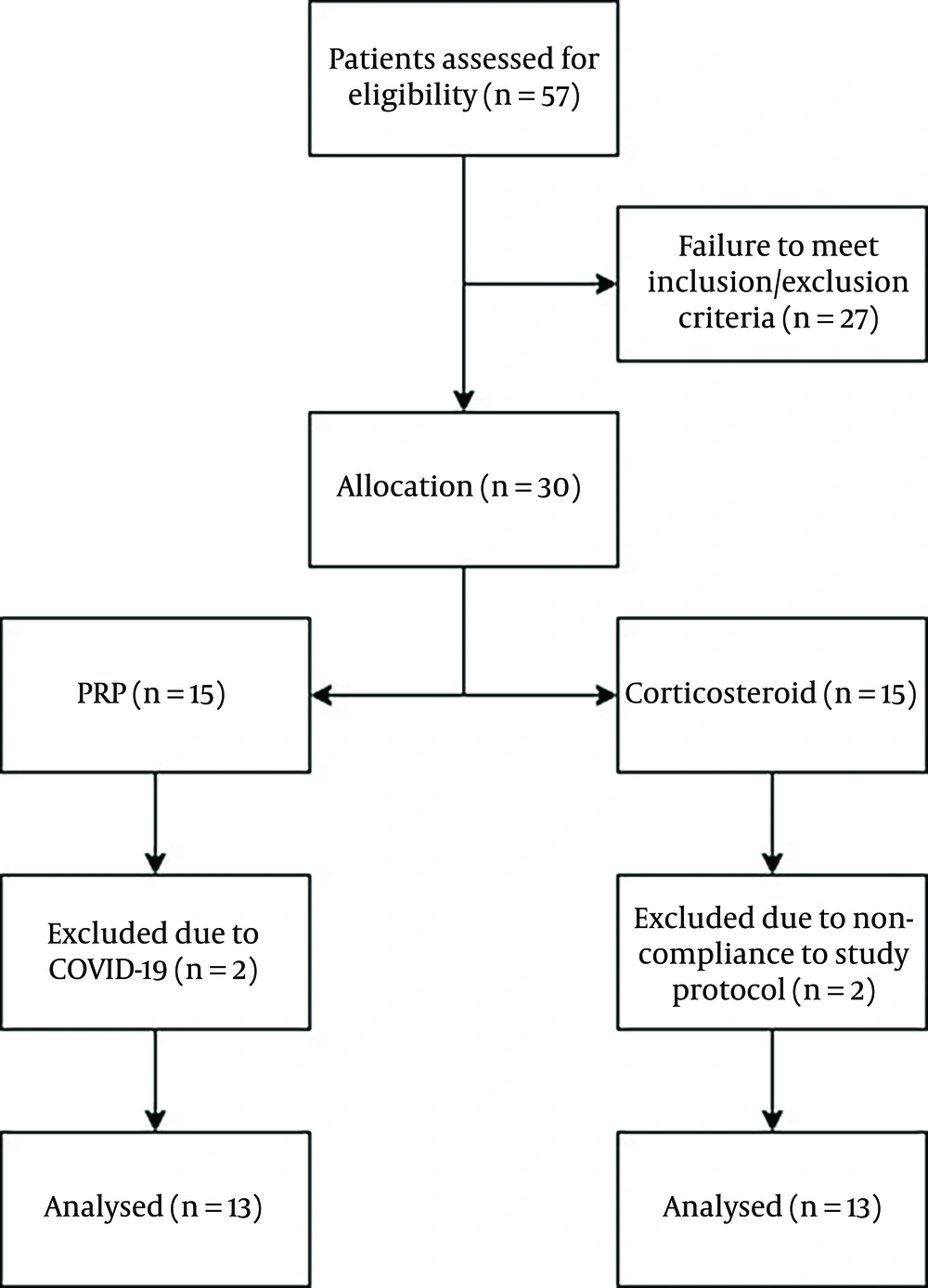
Initially, 30 patients were randomly assigned to participate in two groups (PRP vs. corticosteroids, 15 each). However, during the course of the study, two patients in the PRP group and two patients in the corticosteroid group were excluded due to loss to follow-up. Ultimately, 13 patients in the PRP group (mean age: 44.07 ± 8.21 years) and 13 patients in the corticosteroid group (mean age: 44 ± 9.85 years) were included in the analysis. Table 1 demonstrates the baseline characteristics of the patients, and Figure 3 illustrates the CONSORT flow chart of this study. The two study groups did not differ regarding baseline characteristics and were matched for age, gender, Body Mass Index (BMI), duration of symptoms, and the affected foot (all P-values > 0.05).
| Variables | PRP Group (N = 13) | Corticosteroid Group (N = 13) | P-Value |
|---|---|---|---|
| Gender | 0.691 | ||
| Female | 5 (38.5) | 6 (46.2) | |
| Male | 8 (61.5) | 7 (53.8) | |
| Age (y) | 44.07 ± 8.21 | 44 ± 9.85 | 0.983 |
| BMI (kg/m2) | 28.25 ± 3.04 | 28.29 ± 2.81 | 0.971 |
| Symptoms duration (months) | 9.15 ± 5.84 | 8.84 ± 5.41 | 0.983 |
| Affected foot | 0.185 | ||
| Right | 11 (84.6) | 8 (61.5) | |
| Left | 2 (15.4) | 5 (38.5) |
Abbreviations: PRP, platelet-rich plasma; BMI, Body Mass Index.
a Values are expressed as No. (%) or mean ± SD.
4.2. Numeric Pain Rating Scale
As demonstrated in Table 2, the results of the Mann-Whitney tests showed no statistically significant difference in the average pain intensity based on the NPRS between the corticosteroid and PRP groups before the intervention (P = 0.094) and three months after the intervention (P = 0.174). However, NPRS was significantly lower in the corticosteroid group than in the PRP group one month after the intervention (P = 0.003). Additionally, the results of repeated measures showed that the average NPRS score in both the corticosteroid and PRP groups significantly decreased at all follow-up visits compared to the previous NPRS assessment (P < 0.001).
| Time | PRP (N = 13) | Corticosteroid (N = 13) | Difference | P-Value |
|---|---|---|---|---|
| NPRS score | ||||
| Before intervention | 7.76 ± 0.92 | 7.07 ± 0.95 | 0.69 | 0.094 |
| One month after | 5 ± 1.22 | 3.23 ± 1.30 | 1.76 | 0.003 |
| Three months after | 3 ± 1.68 | 3.69 ± 1.43 | -0.69 | 0.174 |
| P-value | P < 0.001 | P < 0.001 | ||
| FADI score | ||||
| Before intervention | 49.3 ± 10.11 | 54.84 ± 8.33 | 5.53 | 0.141 |
| One month after | 64.53 ± 13.96 | 78.69 ± 11.32 | 14.15 | 0.009 |
| Three months after | 78.15 ± 16.76 | 75.76 ± 1196 | 2.38 | 0.68 |
| P-value | P < 0.001 | P < 0.001 | ||
| PFT | ||||
| Before intervention | 5.43 ± 0.91 | 5.03 ± 0.62 | 0.4 | 0.284 |
| One month after | 5.01 ± 0.86 | 4.69 ± 0.53 | 0.32 | 0.486 |
| Three months after | 4.38 ± 1.04 | 4.51 ± 0.59 | 0.13 | 0.121 |
| P-value | 0.006 | 0.014 |
Abbreviations: NPRS, Numeric Pain Rating Scale; FADI, Foot and Ankle Disability Index; PFT, plantar fascia thickness.
4.3. Foot and Ankle Disability Index
As shown in Table 2, the results indicated that there was no statistically significant difference in the average FADI score between the corticosteroid and PRP groups before the intervention (P = 0.141) and three months after the intervention (P = 0.68). However, one month after the intervention, the FADI score was significantly higher in the corticosteroid group than in the PRP group (P = 0.009). Additionally, the results of repeated measures showed that the average FADI score in both the corticosteroid and PRP groups significantly increased at all follow-up visits compared to the previous FADI assessment (P < 0.001).
4.4. Roles and Maudsley Score
As shown in Table 3, one month after the intervention, the majority of participants in the PRP group reported an average level of satisfaction (level 3), while the majority of participants in the corticosteroid group reported a good level of satisfaction (level 2). However, Fisher's exact test revealed no statistically significant difference between the two groups (P = 0.06). Three months after the intervention, the majority of patients in the PRP group reported a good level of satisfaction (level 2), while the majority of patients in the corticosteroid group reported an average level of satisfaction (level 3). Once again, Fisher's exact test did not reveal any statistically significant difference between the two groups (P = 0.359).
| Time | PRP (N = 13) | Corticosteroid (N = 13) | P-Value |
|---|---|---|---|
| After one month | 0.06 | ||
| Level 1 | 0 | 1 (7.7) | |
| Level 2 | 4 (30.8) | 6 (46.2) | |
| Level 3 | 9 (69.2) | 3 (23.1) | |
| Level 4 | 0 | 3 (23.1) | |
| After three months | 0.359 | ||
| Level 1 | 3 (23.1) | 1 (7.7) | |
| Level 2 | 7 (53.8) | 6 (46.2) | |
| Level 3 | 3 (23.1) | 4 (30.8) | |
| Level 4 | 0 | 2 (15.4) | |
| P-value | 0.228 | 0.123 |
Abbreviation: PRP, platelet-rich plasma.
a Values are expressed as No. (%).
4.5. Plantar Fascia Thickness
As shown in Table 2, the results revealed no statistically significant difference in the average thickness of the plantar fascia between the corticosteroid and PRP groups prior to (P = 0.284), one month after (P = 0.486), and three months after the intervention (P = 0.121). However, repeated measures revealed a statistically significant decrease in the average PFT in each group after the intervention (P = 0.006 and 0.014). Additionally, the distribution of patients with a PFT less than or greater than 4 mm did not differ significantly between the PRP and corticosteroid groups one and three months after the intervention.
5. Discussion
The purpose of this study was to investigate and compare the efficacy of PRP and corticosteroid injections in the treatment of PF over a 3-month period. The results demonstrated that both treatment methods substantially decreased the NPRS score, improved FADI, and decreased PFT within three months. These results suggest that corticosteroids produce rapid and satisfactory short-term effects in patients, but as time passes, these positive effects diminish and the symptoms reappear, in contrast to patients treated with PRP. Our results may indicate that the effects of PRP are produced gradually and slowly but are more long-lasting. However, the results were not statistically different between the study groups.
Similar to our findings, a meta-analysis by Herber et al. (19) on 21 randomized controlled trials (consisting of 1356 patients) concluded that PRP injection was only superior to corticosteroid injection in improving one scale (The AOFAS scale), while there were no differences regarding other scoring systems (PFT and Foot Function Index). A meta-analysis by Alkhatib et al. (20) showed that although the AOFAS score significantly improves in the PRP group compared to corticosteroid injection, there is no difference in terms of FADI and RMS scores between the two treatment options. However, since PRP is considered a safer option than corticosteroids, many clinicians may use it more frequently in clinical settings, even though there may be no significant difference in the outcomes.
On the other hand, another meta-analysis by Seth et al. (21) on 18 studies (comprising 1180 patients) showed that PRP leads to significantly better pain relief and foot function compared to corticosteroids at both the three- and six-month marks. These inconsistencies in the results of meta-analyses indicate that further studies with larger sample sizes and more heterogeneous methodologies are required in this field.
One of the key findings of the present study was that both treatments were effective after one month, but PRP was more effective than corticosteroids after three months. Ugurlar et al. (22) demonstrated that corticosteroids are more effective for up to three months, whereas PRP is more effective from three to twelve months. Another intriguing discovery of the Ugurlar study was that, over a period of 36 months, there were no differences between the groups. Jain et al. (23) reached the same conclusion as this study, finding that PRP is more effective than corticosteroids in a 3-month follow-up, despite using 80 mg of methylprednisolone as a treatment. Similar findings were observed by Sathyendra et al. (24) and Vellingiri et al. (25), whose clinical trials showed that PRP injection leads to superior results compared to local corticosteroid injection. In an intriguing study conducted by Sherpy et al. (26), it was determined that the PRP group showed greater improvement over a 1.5-month period, with no distinctions between the two groups after three months. This distinction may be due to the use of 80 mg triamcinolone for the corticosteroid group and 3 cc PRP. According to Aksahin et al. (27), there is no significant difference between using corticosteroids or PRP to treat PF. This study's results may differ from those of the Aksahin study due to differences in the dosage of methylprednisolone and the volume of PRP used. In 2013, Tiwari and Bhargava (28) demonstrated that PRP might be more effective than corticosteroids even over a one-month period. This superior result may be due to the use of 5 cc PRP, which is 3 cc more than in this study. Additionally, according to the results of other studies, PRP is superior to corticosteroids over the long term because the effects of corticosteroids diminish over time (29-31). For instance, a meta-analysis by Hohmann et al. (32) (including 15 original investigations) demonstrated that corticosteroid injection has no advantage over PRP injection in the short term. However, since most original investigations in this field were of low quality or had a high risk of bias, further studies are needed to validate these findings. The sample size of our study is a limitation, potentially impacting the generalizability and statistical power of our findings. Future studies should aim to include a larger cohort of participants to enhance the robustness of the results. Additionally, multi-center trials could provide more comprehensive data by encompassing a broader demographic and clinical spectrum. Extending the follow-up period would also be beneficial in assessing the long-term efficacy and safety of the treatments. By addressing these aspects, future research can build upon our findings and contribute more substantially to the understanding and management of PF.
5.1. Conclusions
Within three months, both corticosteroid injection and PRP treatment significantly decreased NPRS scores, improved FADI, and decreased PFT. Additionally, our results demonstrated that the effects of corticosteroids in PF began to diminish after one month, whereas in patients treated with PRP, these effects continuously increased and were more long-lasting over the three-month period.