1. Background
The role of oxidative stress (OS) in exercise has been a subject of scientific investigation since the 20th century, when the presence of radicals in living cells was initially reported (1-3). This observation spurred extensive research into free radicals (FrRls) and cellular oxidation-reduction equilibrium (4, 5), leading to the development of a new field focused on FrRls and the interplay between OS and cellular reduction (2). In the late 1970s, theories connecting muscular exertion to increased oxidative damage began to emerge, laying the groundwork for contemporary exercise physiology. Numerous studies have since investigated the effect of exercise on markers of OS in athletes (5). These studies suggest that prolonged (6, 7) or high-intensity (8, 9) exercise may elevate FrRls in active skeletal muscles, contributing to the development of OS.
Physiologically, OS represents an imbalance between FrRls and antioxidant (AntOx) status, leading to potential cellular damage, including lipid peroxidation and DNA damage (10). This imbalance is exacerbated during intense physical activity, resulting in inflammation, muscle fatigue, and reduced muscle function (11). However, exercise-induced OS can also stimulate adaptive responses, promoting muscle adaptation and improved performance over time (12). Understanding the role of OS in physical activity is widely acknowledged in sports medicine, with substantial research expanding in this area over time (2). FrRls generated during aerobic cellular metabolism are crucial regulators of signaling processes. The relationship between exercise and OS is complex and depends on the mode, intensity, and duration of exercise (11). Regular training at moderate intensity reduces OS and improves health, while intense exercise can increase OS indicators. Muscle damage from severe exercise intensity refers to the structural and functional changes in muscle tissue (13).
Muscle damage, often observed in prolonged (14) or high-intensity (15) exercise, refers to structural and functional changes in muscle tissue. This damage is closely linked with oxidative stress (OS) due to the generation of reactive oxygen species (ROS) during physical activity. These ROS can harm cellular components and contribute to symptoms such as inflammation and muscle dysfunction (16). Cellular components, including lipids and DNA, can be affected by OS (5). However, it is essential to note that exercise-induced OS can also trigger adaptive responses and promote muscle adaptation (3, 17). Evidence from past studies indicates that various exercise protocols, including high-intensity (18-20) and prolonged (21-23) exercise, can increase muscle damage indicators. For instance, research by Lukas Cipryan and Leite et al. demonstrated that high-intensity training protocols in moderately trained males significantly increased muscle damage and OS biomarkers immediately post-exercise (24). Similarly, studies by Paschalis et al. and Tzatzakis et al. highlighted significant changes in OS and muscle damage markers following endurance exercises (23, 25).
The plasma concentration of F2–Isoprostanes (ISO) increased approximately 1.6-fold in response to activity but returned to pre-exercise levels within the first hour of recovery. In a study by Steensberg et al., eleven healthy male participants exercised on a treadmill for 2.5 hours, and the researchers reported a similar decrease in ISO levels after prolonged activity (26). Burt et al. investigated the effects of exercise-induced muscle injury on physiological and metabolic responses before, during, and after submaximal running (27). They observed that endurance exercise increased muscle soreness and creatine kinase (CK) concentrations as indicators of muscle injury. Additionally, He and Zhang reported an increase in CK following endurance exercise (28). However, other studies investigating the impact of exercise on OS and muscle damage have shown varying findings (2, 22, 29).
The consumption of local foods and nutritional products before, during, and after exercise has been explored to enhance athletic performance (30). This approach aims to optimize performance and promote cognitive improvement by advocating the consumption of locally sourced foods alongside exercise. Implementing a diet plan that includes dietary supplements is essential for addressing nutritional deficiencies (31), enhancing athletic performance (32), and facilitating effective exercise recovery (33). Natural and healthy supplements are typically less processed than imported foods, and it is fiscally prudent to consume them as dietary supplements rather than opting for more expensive alternatives (34). Organic and locally sourced foods, such as carbohydrates (CHO), proteins (PRO), and antioxidants (AntOxs), generally offer more nutrients.
Sago (Sa) and soy (So) are two locally consumed sources of carbohydrates (CHO) and protein (PRO) in Southeast Asia, valued for their nutritional benefits. Sa contains 88% CHO in the form of the polysaccharides amylose and amylopectin, while So PRO is a combination of several essential amino acids, including leucine, isoleucine, and valine (35). Sa and So are widely used in traditional regional dishes and local cookies. Unlike cultures where potatoes or grains are staple foods, Sa is a primary food source. In addition to potatoes, rice, and maize, people from these cultures incorporate Sa into their pasta dishes.
Numerous studies have examined the impact of isocaloric CHO-PRO supplementation on endurance performance. The goal of matching the energy content of CHO and PRO supplements is to investigate their effects on performance and recovery beyond just calorie content. Romano-Ely et al. compared CHO-PRO and isocaloric CHO supplements on muscle damage and fatigue (36). They concluded that biomarkers associated with muscle damage decreased after exercise in the CHO-PRO condition, unlike the isocaloric CHO treatment. Abdul Manaf et al. conducted a study where cyclists were administered an isocaloric supplement comprising Sago+Soy (SS), Sa, or a sports drink. The supplementation, providing 24 kcal per 100 mL, was given to participants at 4 mL per kg of body weight in beverages at 30-minute intervals. They found no significant changes in creatine kinase (CK) after 24 hours of exercise (37). Samaras et al. investigated the effects of CHO-PRO consumption on oxidative stress biomarkers during endurance exercise. Their study showed that CHO-PRO supplementation significantly reduced oxidative stress after exercise (38). Meanwhile, Kerasioti et al. examined the effects of CHO-PRO intake on oxidative stress markers following exhaustive cycling (39). They concluded that CHO-PRO and CHO supplements did not affect oxidative stress levels during heat exercise.
To the best of our knowledge, no studies have investigated the potential differences in the effects of Sago (Sa) (CHO in its local form), Soy (So) (PRO in its local form), and isocaloric Sago+Soy (SS) (CHO-PRO combination) on biomarkers associated with muscle damage and oxidative stress (OS) during endurance exercise conducted under hot environmental conditions. This work builds on previous studies by examining the impact of consuming isocaloric Sa and So on muscle damage and oxidative stress indicators. Notably, this study is the first to explore these effects in a hot and humid environment (~31°C; 70% relative humidity).
2. Objectives
The objectives of the current study are to assess the influence of CHO-PRO supplementation in the form of locally available Sa (CHO), So (PRO), and isocaloric SS (CHO-PRO combination) on plasma levels of creatine kinase (CK) and F2-isoprostanes (ISO) biomarkers immediately after endurance cycling performance in the heat and 24 hours post-exercise, compared to a placebo (PL).
3. Methods
The current study utilized a randomized, single-blind, placebo-controlled crossover design to investigate the effects of isocaloric Sago (Sa) and Soy (So) supplementations on indicators of muscle damage and oxidative stress (OS) in relation to cycling performance.
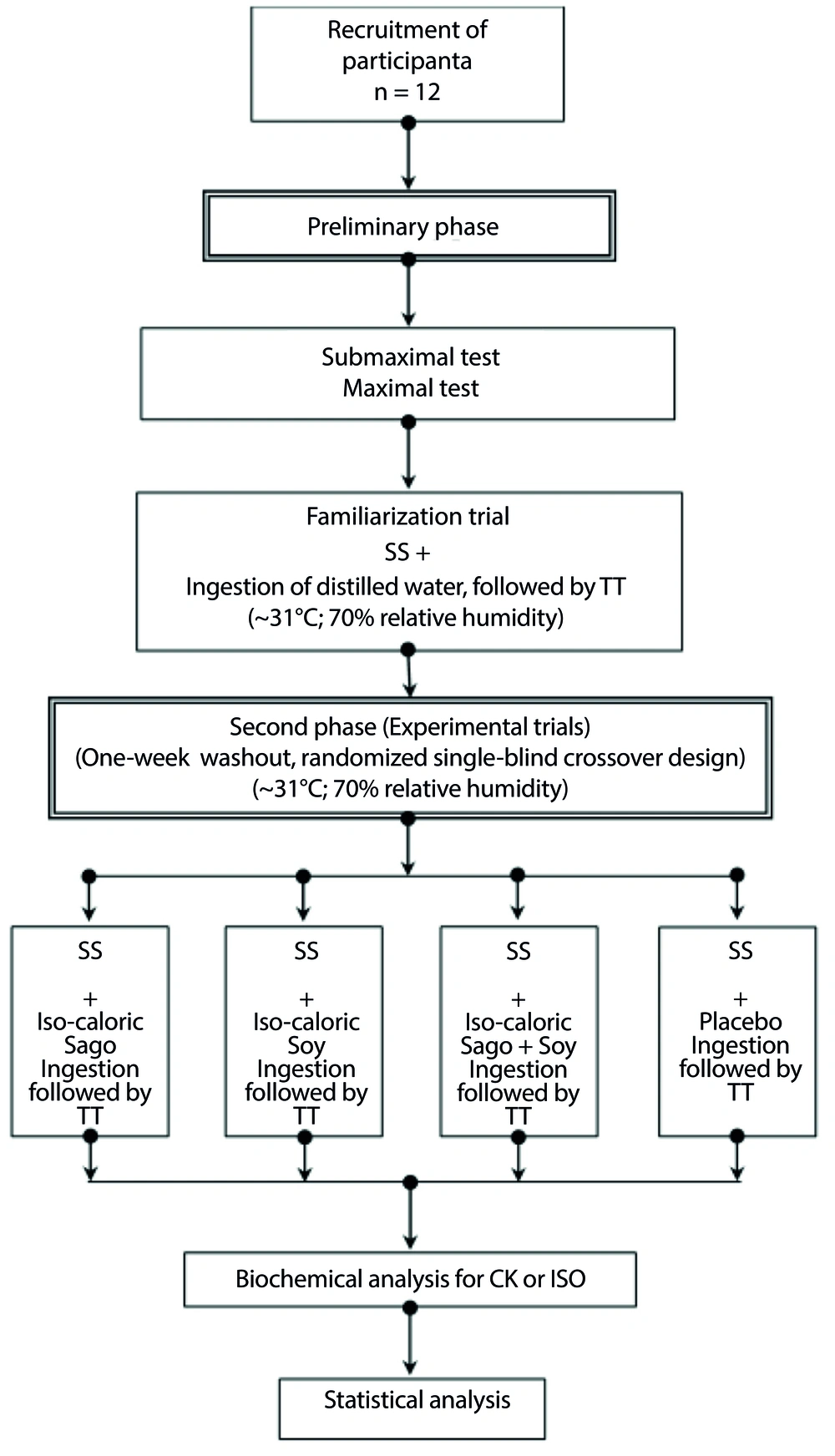
The sample size was determined using PS Power and Sample Size Calculation v.2.1.30. Twelve moderately trained, heat-adapted male cyclists from the Kelantan cycling team were randomly selected to participate. These athletes were accustomed to the tropical environment, competing in regional cycling races and training approximately 80 km three times weekly prior to the study. They were randomly assigned to one of four supplement groups: Sa, So, SS, or placebo (PL), across four separate trials in a crossover design. Although the sample size of twelve was deemed sufficient for this study, a larger sample would enhance the statistical power and generalizability of the results. Increasing the sample size in future studies could lead to more definitive conclusions and potentially reveal subtler effects of the interventions. The physical characteristics of the participants are detailed in Table 1.
| Variables | Mean ± SD |
|---|---|
| Age (y) | 19.0 ± 5.6 |
| Height (cm) | 170.8 ± 7.6 |
| Body weight (kg) | 60.1 ± 11.2 |
| Body fat (%) | 16.6 ± 4.4 |
| Body Mass Index (kg.m-2) | 20.5 ± 3.0 |
| Maximal oxygen consumption (mL.kg-1.min-1) | 56.5 ± 6.5 |
| Maximum heart rate (beats.min-1) | 201 ± 5.6 |
The Physical Characteristics of the Participants (N = 12)
Each participant received a comprehensive explanation of the research methodologies and associated risks. To determine their eligibility, participants were required to read and sign an informed consent form. This study was funded by the e-ScienceFund, administered by the Malaysian Ministry of Science, Technology, and Innovation (MOSTI). The project is identified as USM/0000814 and is associated with the Sports Science Unit at the Health Campus of Universiti Sains Malaysia. Additionally, this study was registered as a clinical trial (register ID: IRCT20190906044711N1).
3.1. Supplementations of the Study
The Sa and So protein isolate flours were purchased from Sim Company Sdn. Bhd. in Penang, Malaysia. The supplements provided to participants were designed to have equal calorie content, estimated to be around 300 kcal. The consumption of these supplements was randomized and administered five times at designated intervals (0, 20, 40, 60, and 80 minutes) during 90 minutes of steady-state cycling. All supplements were prepared simultaneously before each trial and stored at room temperature (25°C). The taste of all supplements and the placebo (Table 2) was made identical by incorporating 5 mL of non-caloric chocolate flavor (Star Brand, Selangor, Malaysia) into 1000 mL of each drink (40-42). Distilled water was used to prepare the placebo, as it contained no caloric substance. This study represents the first investigation into the influence of isocaloric Sa and So supplements on exercise in a hot and humid environment (31°C; 70% relative humidity), with all conditions providing equivalent caloric intake.
| Type of the Supplement | Amount (mL) | CHO (g) | PRO (g) | Concentration (%) | Total Energy (kcal) | |
|---|---|---|---|---|---|---|
| CHO | PRO | |||||
| Content per serving consumed during each experimental trial | ||||||
| Sago | 200 | 15 | 0 | 7.5 | 0 | 60 |
| Soy | 200 | 0 | 15 | 0 | 7.5 | 60 |
| Sago+Soy | 200 | 12 | 3 | 6.0 | 1.5 | 60 |
| Placebo | 200 | 0 | 0 | 0 | 0 | 0 |
| Content of total consumed during each experimental trial | ||||||
| Sago | 1000 | 75 | 0 | 7.5 | 0 | 300 |
| Soy | 1000 | 0 | 75 | 0 | 7.5 | 300 |
| Sago+Soy | 1000 | 60 | 15 | 6.0 | 1.5 | 300 |
| Placebo | 1000 | 0 | 0 | 0 | 0 | 0 |
The Basic Nutrient Composition of the Supplements (42)
3.2. Trials of the Study
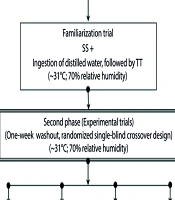
The exercise protocol used in the trials was designed to simulate a real competition. Each participant ingested Sa and So isocaloric supplements, as outlined in Table 2, during the prolonged exercise. The study was conducted in two distinct phases: The preliminary trials and the experimental trials. The preliminary phase included three trials, as depicted in the method flowchart in Figure 1: The submaximal test, the maximal test, and the familiarization trial. These trials aimed to select and familiarize participants with the procedures for the second stage of the study.
The objective of the submaximal test was to determine the relationship between pedaling exertion and VO2 in the participants. The data obtained were then used to estimate the required exertion for 90 minutes of steady-state cycling at each individual’s VO2max. Participants cycled for 16 minutes at a constant cadence of 60 RPM with varying workloads of 50, 80, 110, and 140 watts while pedaling in the laboratory. They subsequently underwent a maximal test to determine their VO2max using an electronically braked ergometer (Excalibur Sport, Lode, The Netherlands). In the familiarization trial, which resembled the subsequent experimental trials, participants refrained from consuming the Sa and So supplements provided in the experimental trials. Instead, they were administered distilled water at 20-minute intervals (40). Participants who did not pass this trial were excluded from the study.
During the familiarization and experimental trials, a Digital Psychrometer (Extech Instrument RH300, USA) was used to measure the room temperature and relative humidity (31°C; 70% relative humidity). The chamber temperature was maintained at 31°C using halogen lighting (Philips-500 W, France) and an air conditioner (York®, Malaysia). Relative humidity in the chamber was controlled at 70% using a heated water bath (Memment, Germany).
In the chamber, a stationary fan set to speed 2 was used to simulate airflow in an open-air environment by directing airflow toward the subjects. Before each trial, participants were instructed to refrain from consuming any additional supplements or engaging in any activities for 24 hours. Each participant was provided with a food diary to record their diet for the three days preceding each experimental trial. All participants were instructed to arrive at the laboratory after fasting overnight for 10 to 12 hours. Additionally, participants were asked to follow the same diet plan before the second trial to minimize fluctuations in their blood substances.
Upon their arrival at the laboratory for the experimental trials at 7:30 a.m., participants were given a standard breakfast consisting of two pieces of white bread (Gardenia®, Malaysia) and 250 mL of 8°C distilled water. Approximately 20 minutes after the first blood draw, participants were exposed to a thermally stressful environment in a heat chamber. Each experimental trial began with a 5-minute warm-up at an intensity equal to 50% of their VO2max. Following the warm-up, participants pedaled for 90 minutes at an intensity equivalent to 60% of their VO2max. This cycling activity was followed by a 20-km time trial performance (TT) on a different bike after 5 minutes of passive rest (One Series Aluminium, Trek Road Bikes, USA). To simulate real-world conditions and accelerations for endurance cycling in the heat chamber, this bicycle was held vertically by a device (CycleOps Power JetFluid Pro Trainer, USA). During this time, participants could control their cycling speed using a digital cyclometer (Cateye Strada Wireless, Japan).
3.3. Biochemical Analysis of Muscle Damage and Oxidative Stress Markers
The subjects were cannulated with an indwelling cannula inserted into a subcutaneous forearm vein before eating breakfast. For each blood sample, approximately 0.8 mL of heparinized saline was added to the extension tube to maintain blood flow. Blood samples were collected 30 minutes before exercise, immediately following exercise, and 24 hours after exercise. For CK and ISO determinations, 5 mL of blood was extracted using a sterile 10 mL syringe and transferred to yellow-colored tubes (5 mL, Gel and Clot Activator, 13×100 mm, ST750CG) for each blood withdrawal. Blood samples were centrifuged in sodium heparin tubes (Hettich–Rotina 46 RS, Germany), and plasma was aliquoted into cryotubes and stored at –80°C (Heto Ultra Freeze 3410, Denmark) until biomarker analysis.
The CK concentration was determined using the N-acetyl-L-cysteine (NAC) method. CK, also known as creatine phosphokinase (CPK), is an enzyme secreted by many cells that accelerates the transfer of a high-energy phosphate group from creatine phosphate to ADP. Following ATP synthesis, the phosphate group is used to phosphorylate glucose, forming glucose-6-phosphate (G-6-P) in the presence of hexokinase. G-6-P is then oxidized by glucose-6-phosphate dehydrogenase (G-6-PDH), which also reduces nicotinamide adenine dinucleotide phosphate (NADP) to its reduced form, NADPH. The rate of NADPH synthesis is observed at 340 nm and is related to the CK activity in the sample. NAC acts as an enzyme reactivator during these processes. CK concentrations were measured at 340 nm using a commercial reagent (7D63–21, Creatine Kinase Reagent) and an automatic analyzer (Abbott Aeroset C–8000, USA). The CK activity was quantified in units per liter (U/L-1), with the calculation based on the minimum volume of reagent required per kit.
The presence of ISO (8-iso Prostaglandin F2α) in plasma was determined using an enzyme immunoassay (EIA) technique, specifically the enzyme-linked immunosorbent assay (ELISA) method. The concentration of ISO was measured using reagents from Cayman Chemical's ACE™ EIA Kits (Catalog No. 516351, USA). After defrosting the plasma, the concentration was assessed using a photometric microplate reader (Molecular Devices; Versamax Tunable Micro Plate Reader, USA). A total of 50 µL of plasma was added to sample wells that had been pre-coated with mouse anti-rabbit antibody. Standards were prepared at eight different concentrations specified in the test protocol, with 50 µL of each standard added to the standard wells. Then, 50 µL of ISO AChE Tracer was introduced into every well, except for the Total Activity and Blank wells. Each well, except for the Total Activity, Non-Specific Binding, and Blank wells, received 50 µL of ISO EIA Antiserum. The plate was covered with plastic film and incubated at 4°C for 18 hours. After incubation, the wells were emptied and rinsed five times with Wash Buffer. Following this, 200 µL of Ellman's Reagent was added to each well, and 5 µL of tracer was added to the Total Activity well. The plate was then covered with plastic film and allowed to develop in the dark at room temperature for two hours on a microtitre plate mixing apparatus (IKA Vibrax® VXR basic, United States). Finally, the plate was measured at a wavelength between 405 and 420 nm.
3.4. Statistical Analysis
IBM SPSS Statistics v.27.0.1 for the Windows operating system was used to conduct the statistical analyses. The data were presented as means and standard deviations (SD), with a significance level set at (P < 0.05). The Shapiro-Wilk test was employed to assess the normality of the data. A repeated measures analysis of variance (ANOVA) was used to evaluate the temporal variations in CK and ISO variables across four consecutive experimental sessions. A paired t-test was used to determine the mean differences between time points, including 30 minutes prior to exercise, immediately after exercise, and 24 hours after exercise. This investigation aimed to determine the effects of Sa, So, SS, and PL on CK and ISO biomarkers following the exercise.
4. Results
As shown in Figure 2, the data for the four trials (Sa, So, SS, and PL) illustrate the variations in CK levels determined after 90 minutes of steady-state cycling and the 20-km time trial (TT) performance. Following the 20-km cycling, CK levels significantly increased in all trials (PL: P = 0.001), Sa: P = 0.05, So: P = 0.05, SS: P = 0.025) compared to baseline. The CK levels at the end of the 20-km cycling were 230.0 ± 114.2 U/L for PL, 282.6 ± 258.1 U/L-1 for Sa, 212.2 ± 126.2 U/L-1 for So, and 260.1 ± 203.4 U/L-1 for SS.
After 24 hours post-exercise, CK levels were significantly lower compared to immediately post-exercise in all conditions (PL: P = 0.005, Sa: P = 0.046, So: P = 0.048, SS: P = 0.05). The CK levels 24 hours after exercise were 171.0 ± 65.0 U/L-1 for PL, 217.7 ± 166.2 U/L-1 for Sa, 192.3 ± 112.0 U/L-1 for So, and 200.2 ± 113.0 U/L-1 for SS. Additionally, CK levels in the SS trial were significantly lower post-20-km cycling (P = 0.025), which might indicate an improvement in the recovery of muscle damage.
The unexpected similarity in CK reduction between the placebo and supplementation groups warrants further examination. Several factors could contribute to this finding. First, the placebo effect itself might have had a significant impact, given the participants' awareness of being part of a study, which could influence their physiological responses. Second, the rigorous environmental conditions (heat and humidity) might have enhanced the body's natural recovery processes, thereby minimizing the differences between the PL and supplementation conditions. Lastly, individual variations in response to supplementation and exercise stress could have masked the effects of the supplements.
Figure 3 shows the variations in ISO levels after 90 min of steady-state cycling and the TT for each supplementation trial. Following the 20-km cycling, ISO levels were significantly reduced in all four trials (< 0.001) compared to baseline. The ISO levels at the end of the 20-km cycling were 393.0 ± 21.0 pg/mL-1 for PL, 424.1 ± 49.0 pg/mL-1 for Sa, 403.4 ± 35.1 pg/mL-1 for So, and 429.0 ± 41.0 pg/mL-1 for SS.
After 24 hours, ISO levels were significantly lower than baseline levels and those immediately post-20-km cycling (P < 0.001) and (P = 0.001), respectively). The 24-hour ISO levels were 398.5 ± 18.5 pg/mL-1 for PL, 402.4 ± 55.2 pg/mL-1 for Sa, 388.4 ± 64.4 pg/mL-1 for So, and 394.0 ± 62.4 pg/mL-1 for SS. In the Sa and SS trials, ISO levels were significantly lower compared to the post-exercise 20-km cycling performance (Sa: P = 0.001, SS: P = 0.05). The similar reductions in ISO levels across all trials, including the PL, suggest that factors other than the supplements might be influencing the results. These factors include the high heat and humidity, which likely activated the body's antioxidant defense mechanisms, contributing to the reduction in oxidative stress markers regardless of supplementation. Additionally, adequate hydration and controlled dietary intake before the trials, which help maintain plasma glucose levels and indirectly reduce oxidative stress, should not be overlooked.
These findings are consistent with recent studies suggesting that CHO and PRO supplementation does not always significantly improve muscle damage and oxidative stress markers during prolonged exercise. This indicates that while supplementation may offer some benefits, other factors such as environmental conditions, individual physiological responses, and psychological influences can significantly affect the results.
5. Discussion
The current study represents the first investigation into the impact of isocaloric Sa and So supplements on muscle damage and oxidative stress (OS) indicators under hot and humid conditions, characterized by a temperature of 31°C and a relative humidity of 70%. This pioneering study indicated that isocaloric Sa and So supplements affected the biomarkers CK and ISO immediately following the time trial (TT) and 24 hours after exercise, which measure muscle damage and OS, respectively.
Both prolonged exercise (27, 43, 44) and high intensity (45-48) have been correlated with increased tissue damage (49). All experimental trials showed significant increases in CK levels after cycling performance (27, 46, 47, 50). Consistent with previous research, there was a substantial reduction in CK levels 24 hours after cycling compared to the levels observed immediately following the TT (27, 46, 47, 50-53). However, the low CK levels in the placebo (PL) group indicated that Sa, So, and SS supplementation did not significantly prevent muscle damage. This surprising finding supports prior research showing that CHO and PRO feedings did not improve CK levels during extended exercise. Physiological stimuli, such as physical activity, significantly impact oxidative stress (49, 54). The current study observed a significant reduction in ISO after the TT and 24 hours of exercise, consistent with previous studies. This supports evidence indicating that prolonged physical activity leads to a reduction in ISO levels (48, 49, 54, 55). The reduction of ISO in the PL group, which was an unexpected result, suggested that the addition of Sa, So, and SS did not improve OS status. The body's antioxidant defensive mechanisms may activate during prolonged heat-induced exercise. However, limited research suggests that many factors contribute to the drop in ISO. Moreover, in endurance athletes, adequate hydration and antioxidant supplementation may aid in preventing OS and reducing ISO (56, 57). These findings align with studies indicating that exercise-induced OS can activate adaptive antioxidant responses, reducing muscle damage markers (Pingitore et al., 2015; Finaud et al., 2006) (11, 54). The lack of significant differences between the supplementation and PL groups may also reflect the adequacy of the participants' baseline nutritional status and training adaptations to heat.
A study by Hansen et al. found that CHO-PRO ingestion during cycling for top cyclists did not show noticeable benefits in markers of muscle damage, such as CK, compared to CHO intake alone (58). Similarly, Rowlands et al. indicated that PRO-enriched feeding had no apparent effect on next-day performance or CK blood concentrations (59). According to Romano-Ely et al., CK levels increased during recovery, and there was no difference in performance between CHO-PRO and CHO supplement isocaloric beverages (36). Previous research has shown that the consumption of PRO-CHO supplements is associated with a reduction in muscle damage, which contradicts the findings of the current study. Saunders et al. reported that CHO-PRO consumption during endurance cycling decreased CK levels, as it provided 20% more calories than CHO alone (51). These consistent findings underscore the challenges in achieving substantial improvements in CK levels through nutritional interventions during exercise and highlight the need for further investigation into effective strategies for mitigating muscle damage.
Stress-hormonal responses may be diminished, reducing oxidative stress (OS) when CHO supplementation occurs during exercise (60). The increase in cortisol and epinephrine levels known during endurance exercise may be mitigated by consuming CHOs, which can decrease ROS production (61). Furthermore, Romano-Ely et al. demonstrated that CHO-PRO supplementation may reduce elevated cortisol levels and higher concentrations of catecholamines. This study highlights the more effective isocaloric CHO-PRO, with antioxidant properties compared to CHO alone, and shows that supplementation with CHO-PRO significantly reduced OS following endurance exercise (36). The correlation between the duration and intensity of exercise and the reduction in lipid peroxidation susceptibility in skeletal muscle is additionally supported by the elevation in the activity of antioxidant enzymes during exercise. Antioxidants, which consist of glutathione peroxidase and superoxide dismutase enzymes, are critical for preventing oxidative stress caused by physical activity (62-65).
In a study by Rokitzki et al., oxidative stress (OS) decreased after the end of cycling, showing that antioxidant (AntOx) status increased following marathon recovery (66). According to the current study, the reduced ISO level 24 hours after a time trial (TT) may suggest that lingering oxidant stimuli sustain an extended defense mechanism against OS. While it is essential to consider alternative explanations and additional defense mechanisms that may inhibit the formation of free radicals or reduce their harmful effects, these findings are consistent with studies indicating that training in hot and humid conditions does not increase OS but significantly influences the body's AntOx status (66).
The primary limitations of this study include the small sample size and the potential for uncontrolled confounding variables, such as participants' baseline nutritional status and individual variability in heat acclimatization. These factors may have influenced the observed reductions in muscle damage and OS markers. Despite these limitations, the study provides valuable insights into the role of carbohydrate (CHO) and protein (PRO) supplementation during prolonged exercise in hot environments. Future research with larger sample sizes and controlled baseline conditions is essential to validate these findings and clarify the mechanisms involved.
The findings suggest that athletes may not require specific CHO-PRO supplementation to mitigate muscle damage and OS during prolonged exercise in the heat, as endogenous AntOx defenses and effective hydration strategies might suffice. These results have practical implications for endurance athletes and coaches, emphasizing the importance of adequate training and acclimatization over reliance on supplements. Future research should investigate the long-term effects of different nutritional strategies and explore the underlying adaptive mechanisms in more detail. In summary, this study highlights the complexity of muscle damage and OS responses during exercise, the potential influence of supplementation on CK and ISO levels, and the need for additional research to fully understand the mechanisms underlying OS reduction, particularly in hot and humid environments.
5.1. Conclusions
To our knowledge, this study represents the first investigation into the impact of isocaloric Sa and So supplements on muscle damage and oxidative stress (OS) biomarkers under hot and humid conditions (31°C, 70% relative humidity). These experiments conclusively indicated that the levels of creatine kinase CK and ISO, which serve as biomarkers for muscle damage and OS, experienced substantial reductions immediately after exercise. However, the unexpected observation of a substantial reduction in both biomarkers in the placebo (PL) group suggested that supplementation with Sa, So, and SS did not improve muscle damage and OS, as the PL group behaved similarly. This unanticipated outcome aligns with recent studies indicating that carbohydrate (CHO) and protein (PRO) feedings did not significantly enhance CK and ISO levels during prolonged exercise. Therefore, it emphasizes the complexity of muscle damage and OS responses during exercise and the nuanced influence of supplementation on these indicators. It also underscores the need for further research to comprehensively understand the mechanisms underlying muscle damage and OS reduction, particularly in hot and humid environments.
![Plasma CK changes after experimental trials [* Significantly different from baseline (P < 0.05); ** Significantly different from Post TT (P < 0.01); *** Significantly different from baseline (P < 0.001); + Significantly different from Post TT (P < 0.05)]. Plasma CK changes after experimental trials [* Significantly different from baseline (P < 0.05); ** Significantly different from Post TT (P < 0.01); *** Significantly different from baseline (P < 0.001); + Significantly different from Post TT (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/6737ec2360734fcc529ce51036059219992ad041/asjsm-144084-i002-F2-preview.webp)
![Plasma ISO changes after experimental trials [* Significantly different from Post TT (P < 0.05); *** Significantly different from baseline (P < 0.001); +++ Significantly different from Post TT (P < 0.001)]. Plasma ISO changes after experimental trials [* Significantly different from Post TT (P < 0.05); *** Significantly different from baseline (P < 0.001); +++ Significantly different from Post TT (P < 0.001)].](https://services.brieflands.com/cdn/serve/3170b/47bad3a58f366f2812985b616edda34634bcd4af/asjsm-144084-i003-F3-preview.webp)