1. Background
Throughout puberty, both males and females experience significant hormonal changes and an accelerated accumulation of bone mass (1). This stage is crucial, as the accumulation of bone mass during puberty plays a significant role in determining peak bone mass in adulthood. Inadequate bone mineral mass or density in adulthood can result in osteoporosis and an increased risk of fractures later in life (2). The risk factors for osteoporosis are well-established and include insufficient calcium and vitamin D intake, smoking, alcohol consumption, physical inactivity, early menopause, and family history (3). During the first 20 years of life, the skeleton undergoes substantial growth in both size and density, with more than 50% of peak bone mass being acquired during the teenage years (4).
Growth hormone (GH) plays an essential role in the attainment of peak bone mass and the development of optimal trabecular bone microarchitecture during late adolescence and early adulthood (5). These anabolic effects are key determinants of fracture risk in older age (5). In a study by Dixit et al., bone mineral density increased by 40 - 50% during puberty, mainly due to the expression and activity levels of sex steroid hormones and the GH/IGF axis (6). Additionally, primary hyperparathyroidism and hyperthyroidism can negatively affect bone mass (7). Nutritional deficiencies in calcium or vitamin D can also impair optimal bone mass (8). Approximately 30% of children may experience fractures before adulthood, commonly in the forearm (9).
The attainment of optimal bone mass is influenced by a combination of genetic factors and environmental conditions, such as the amount of stress placed on the skeletal system during adolescence through physical activity (10). During adolescence and early adulthood, physical activity plays a fundamental role in determining peak bone mass and, consequently, long-term bone health (11). Leading physical activity organizations recommend aerobic exercise for fat loss and resistance training to increase bone and lean body mass (12). However, one form of exercise often overlooked is plyometric jump training (PJT), which involves jump drills that utilize the stretch-shortening cycle (13). Plyometric loading is particularly effective in both children and adolescents during periods of bone growth (14). For example, studies have shown that tennis players who trained from childhood have 3 - 4 times higher bone density in their dominant hand than in their non-dominant hand. Similarly, gymnasts who trained for 3 - 4 years, 8 - 10 hours per week, exhibited increased bone mass formation in selected areas of the skeleton (15). It is important to strengthen bones during early childhood to preserve skeletal integrity later in life. The bone response to physical exercise depends on the frequency, duration, and intensity of the exercise, with intensity being the most important factor (13, 14, 16).
Bone health is crucial during growth and puberty, and maintaining an active lifestyle is essential. Plyometric exercises offer a potential therapeutic and preventative approach to bone diseases by positively influencing the critical factors involved in bone development. However, there is limited research on this topic, particularly regarding youths and adolescents.
2. Objectives
Therefore, this study aims to explore the impact of plyometric exercises on male adolescents' serum levels of GH, vitamin D, parathyroid hormone, phosphate, and calcium.
3. Methods
3.1. Subjects
This applied-fundamental study was conducted using a semi-experimental, pre-test and post-test design with a control group (CG). Thirty healthy senior high school boys (mean ± SD; age, 13.68 ± 0.47 years; height, 166.50 ± 9.91 cm; BMI, 22.90 ± 4.33 kg/m²) from one district in Yazd city, who had not taken any special medication and had not engaged in regular sports activities for the past 6 months, voluntarily participated in this study. All individuals were randomly divided into two groups: The plyometric exercise group (n = 15) and the CG (n = 15). To ensure homogeneity among the subjects and reduce the effect of maturity, the deviation of the maturity formula was employed. Written consent was obtained from the volunteer students, and their medical records were reviewed prior to random group assignment. The Ethics Committee of Shahrekord University (IR.SKU.REC.1402.007) approved the study protocols in compliance with the ethical guidelines of the Helsinki declaration, and the study protocol was registered on the Iranian Clinical Trials website (IRCT20221102056381N1). Both participants and their parents provided written consent. To ensure accurate results, blood samples were taken after a 12-hour overnight fast, and participants were instructed to minimize activity 48 hours prior to the tests.
3.2. Anthropometric Measurements
The participants' height was measured while they stood against a wall without shoes, using a standard stadiometer (Seca, Germany), accurate to the nearest 0.1 cm. Body weight was recorded before and after the 12-week sports training program using a digital scale (Beurer, model GS27), accurate to the nearest 0.1 kg. Participants wore minimal clothing and no shoes during the weighing process. Subcutaneous fat in the triceps, upper thigh, and thigh regions was assessed using FAT TRACK PRO digital callipers. Waist circumference was measured at the navel, and hip circumference was measured at the most prominent part of the hips, using a tape measure accurate to within 0.5 cm. Body Mass Index (BMI) was calculated using the formula: Weight (in kilograms) divided by height (in meters) squared. The age at which participants reached peak height velocity (PHV) was determined using Mirwald's formula, which considers physical data and calendar age (17).
3.3. Study Design
Our research aims to investigate the effects of a 12-week plyometric program on the explosive strength performance and hormonal response of male adolescents. Prior to initiating the study protocol, we will thoroughly explain the entire experimental procedure to participants during separate sessions. To evaluate the effects of the training, we employed a between-group design, dividing participants into an experimental group (PG), which followed the plyometric program, and a CG, which continued with their regular Physical Education classes. The plyometric group (PG) participated in plyometric training sessions three times a week for 12 weeks, while the CG did not receive any additional training. Pre- and post-measurements were taken for both groups to assess explosive strength and hormonal response via blood sampling, with all testing conducted simultaneously for both groups.
3.4. Bone Mass Measurements
We utilized dual-energy X-ray absorptiometry (LUNAR bone densitometry, USA) to measure bone mass density (BMD) in various regions of the body, including the lumbar spine (L2-L4), femoral neck, greater trochanter, and the whole body. Our laboratory has a precision error rate of 1.0% for the lumbar spine, proximal femur, and whole-body BMD. The scan length was set to 4.0 inches, and participants lay on the table during the procedure. The femoral shaft bone mineral content (BMC) has an in-house coefficient of variation (CV) of 1.2%. In addition to BMC, we used whole-body scans to determine bone-free lean body mass and fat mass (CV 1.2%). All scans and analyses were conducted by the same skilled technician to ensure consistency.
3.5. Plyometric Training
A 12-week study on plyometric training for adolescents aged 13 - 14 was conducted. The participants were divided into a CG and a PG. The PG received three additional hours of training per week, totaling 36 hours over the course of the study. Pre- and post-tests were conducted on both groups to assess explosive strength, BMD, and blood samples.
The program was structured into four parts, each with a specific purpose. The first part was an introductory segment lasting approximately 5 - 10 minutes, aimed at preparing the body for more demanding physical activity. The second part was a preparatory segment, lasting around 10 - 15 minutes, focusing on warming up the locomotor system, including the muscles, tendons, and ligaments of the lower extremities. The third part, the main segment, lasted for 25 - 30 minutes and was the most demanding part of the program. Finally, the fourth part was a concluding segment, lasting for 8 - 15 minutes, designed to cool the body down and wrap up the session (14).
Each exercise consisted of a minimum of 3 sets and a maximum of 10 repetitions, with a rest period of 45 to 90 seconds between sets (14). The plyometric training in this study included a diverse range of movements such as running, sprinting, one- and two-legged jumps, extensions, deep jumps, deep Swedish mass jumps, and deep station jumps (14). The detailed design of the plyometric training program model can be found in Table 1. To ensure safety and efficacy, all participants were closely monitored by physical education instructors and experienced experts throughout both the testing and training phases.
| Exercises | Week 0 | Week 1 - 3 | Week 4 - 6 | Week 7 - 9 | Week 10 - 12 |
|---|---|---|---|---|---|
| Two-legged Jumping jack 10 m | × 3 | ||||
| Two-legged Hurdle jump 10 m | × 3 | ||||
| Two-legged Lung jump 10 m | × 3 | ||||
| Jumps with one and the other leg 30 m | × 3 | ||||
| Two-legged plyometric box jumps 20 (50 cm) | 3 × 10 | ||||
| Drop leg jumps 30cm | 3 × 10 | ||||
| Two-legged plyometric box jumps (50 cm) | 3 × 10 | ||||
| Two-legged plyometric with one and the other leg jumps (60 cm) | 4 × 10 | ||||
| Two-legged plyometric box jumps (60 cm) | 4 × 10 | ||||
| Two-legged plyometric with one and the other leg jumps (60 cm) | 4 × 10 | ||||
| Two-legged plyometric box jumps (70 cm) | 4 × 10 | ||||
| Two-legged plyometric with one and the other leg jumps (70 cm) | × 4 | × 4 | |||
| Two-legged plyometric box jumps (80 cm) | × 4 | × 4 | |||
| Two-legged plyometric with one and the other leg jumps (80 cm) | × 4 | × 4 |
3.6. Blood Sampling and Measurement
Blood samples were collected from all participants between 7:00 and 9:00 A.M. following an overnight fast. Samples were drawn into vacutainers, placed on ice, and transported to the hospital for processing. The blood was centrifuged, divided into 1 ml aliquots, and stored at -70°C for batch analyses. Growth hormone levels were measured using the radium kit, a highly sensitive and accurate method for detecting GH in biological samples. For measuring IGF-1, the DRG IGF-11600 enzyme immunoassay kit (Germany) was used with the ELISA test method, which is widely employed for the quantitative determination of IGF-1 in serum or plasma samples. Phosphate and calcium levels were determined using Pars Azmoun kits in combination with the spectrophotometric method, a reliable technique that quantifies minerals based on light absorption in biological samples. Levels of 25(OH) D (25-hydroxy vitamin D) were measured using an enzyme immunoassay (ELISA) (Ideal Tashkhis Atieh, Tehran/Iran) (18).
3.7. Statistics
The distribution of data was assessed using the Shapiro-Wilk test. Group differences were evaluated through analysis of variance with repeated measurements, followed by Sidak post hoc tests. All tests were conducted at a significance level of P < 0.05, and data analysis was performed using SPSS statistical software (version 25).
4. Results
Table 2 presents the characteristics, BMC, and BMD of the participants at baseline and after 8 weeks. The majority of participants were in the mid-pubertal maturation stage (Tanner 2 - 3) at both time points. Notably, there was an observed increase in absolute BMC and BMD values from baseline to follow-up in the PG. Additionally, the mean z-scores for height, BMI, body fat percentage, and BMD were within the normal range compared to healthy individuals of the same age.
| Variables | Baseline | Follow up (12-Week) | ||
|---|---|---|---|---|
| Plyometric | Control | Plyometric | Control | |
| Age(y) | 13.68 ± 0.38 | 14.04 ± 0.49 | - | - |
| APHV (y) | 14.79 ± 0.69 | 14.71 ± 0.30 | - | - |
| Height (cm) | 165.20 ± 10.72 | 167.80 ± 9.23 | - | - |
| Weight(kg) | 56.80 ± 15.22 | 61.61 ± 12.99 | 55.80 ± 13.21 b | 62.53 ± 12.61 |
| BMI (kg/m2) | 20.50 ± 3.70 | 22.85 ± 3.58 | 20.27 ± 3.32 b | 22.92 ± 3.50 |
| Fat (%) | 11.49 ± 4.41 | 17.39 ± 4.28 | 11.27 ± 4.03 b | 17.81 ± 4.18 |
| Sargent test (cm) | 31.20 ± 6.22 | 37.40 ± 8.62 | 43.27 ± 5.64 b | 37.21 ± 7.14 |
| Bone mineral density | ||||
| Femoral neck (g/cm2) | 0.765 ± 0.107 | 0.842 ± 0.113 | 0.810 ± 0.079 b | 0.831 ± 0.106 |
| Lumbar neck (g/cm2) | 0.723 ± 0.104 | 0.739 ± 0.085 | 0.764 ± 0.095 b | 0.726 ± 0.084 |
| Total body (g/cm2) | 1.021 ± 0.087 | 1.023 ± 0.112 | 1.052 ± 0.083 b | 1.029 ± 0.103 |
| Calcium (ml/dl) | 9.31 ± 0.64 | 9.45 ± 0.75 | 9.38 ± 0.28 | 9.46 ± 0.35 |
| Phosphate (ml/dl) | 4.39 ± 0.46 | 3.89 ± 0.67 | 5.01 ± 0.29 | 4.67 ± 0.54 |
| PTH (pg/ml) | 60.95 ± 11.98 | 48.90 ± 16.17 | 67.70 ± 12.64 | 57.45 ± 19.11 |
| Vitamin D (ng/mL) | 23.61 ± 6.67 | 28.09 ± 8.33 | 30.34 ± 7.58 | 32.61 ± 10.76 |
a Values are expressed as mean ± SD.
b There was an observed increase in absolute bone mineral content (BMC) and bone mass density (BMD) values from baseline to follow-up in the Plyometric group.
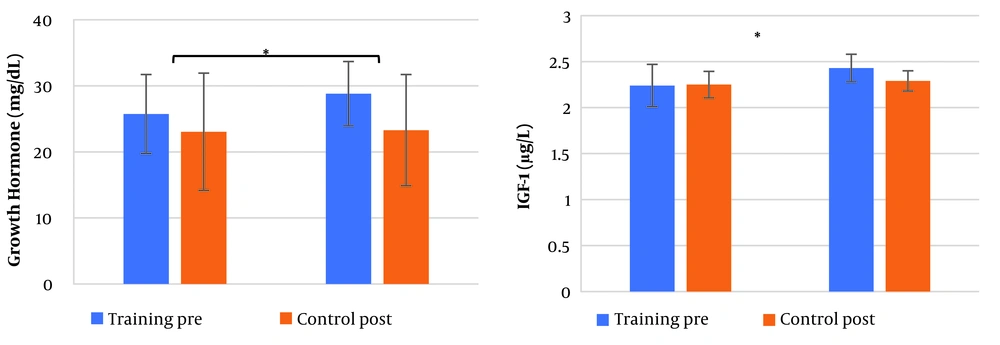
After 12 weeks of training, a comparison of weight, BMI, and fat percentage revealed that the PG had significantly lower values than the CG (as shown in Table 2). Additionally, comparisons of GH (F1,28 = 21.74, P < 0.001, eta = 0.44) and IGF-1 (F1,28 = 6.86, P = 0.014, eta = 0.014) levels between the two groups after 12 weeks of training showed significant changes (Figure 1 a, b), while changes in phosphate (F1,28 = 0.38, P = 0.544, eta = 0.013), calcium (F1,28 = 0.04, P = 0.829, eta = 0.002), and PTH (F1,28 = 0.27, P = 0.605, eta = 0.010) were not significant (Table 2). Vitamin D (F1,28 = 23.33, P < 0.001, eta = 0.45) and vertical jump (F1,28 = 60.41, P < 0.001, eta = 0.68) results revealed that the plyometric training group had significantly higher scores compared to the CG (Table 2).
In terms of BMD, the femoral neck (F1,28 = 20.25, P < 0.001, eta = 0.42), lumbar spine (F1,28 = 18.77, P < 0.001, eta = 0.40), and total body density (F1,28 = 4.24, P = 0.021, eta = 0.21) were significantly higher in the PG compared to the CG after 12 weeks of training (Table 2).
5. Discussion
Recent research reveals that male adolescents who engage in 12 weeks of plyometric training can experience notable improvements in body composition, bone density, and performance. The study focused on exercises specific to plyometric activities to minimize the influence of aerobic or resistance exercises. Notably, most of the boys were in the midst of puberty, which previous research has shown to be a critical period for such training. In a related study, male basketball players in early puberty who completed a two-week lower-body plyometric training program improved their whole-body BMC, ossification markers such as osteocalcin, and physical fitness (19). Meanwhile, a study on adolescent girls found that a more extended period of plyometric jumping exercises during growth could increase peak bone mass, as indicated by trends in bone mass between groups (20).
According to findings from previous studies, performing an average of 33 jumps per day can result in a 3.6% increase in bone mass at the greater trochanter in adult premenopausal women, young adults, and adolescents (20-22). This benefit is unique to jumping and cannot be achieved through running or machine-based weight training. The high impact forces experienced by the hip during jumping may be the key to this improvement. While the femur undergoes compressive forces during landing, muscles attached to the trochanter generate tensile forces during takeoff, weight training, and plyometric jumping. These muscles provide hip joint stability and mobility. Understanding their biomechanics is crucial for optimal physical performance and injury prevention. The squat-type preparation before takeoff may contribute to the bone mass increase in this region of the hip.
The exercise routine in the study included various acceleration and deceleration patterns, which may have introduced unaccustomed stresses on the skeleton. It is also speculated that the greater trochanter is more receptive to loading due to its higher percentage of trabecular bone compared to the femoral neck. The significant differences observed between the training and CGs can largely be attributed to the sedentary nature of the CG. Additionally, it is plausible that exercise training during puberty positively affects bone health. This theory is supported by findings from several studies, which show that adults who engaged in high-intensity physical activity before or around puberty (between 6 - 14 years old) tend to have better health outcomes, such as higher bone mass (10, 21, 23, 24).
Plyometric training is a type of exercise that can significantly improve the jumping ability of adolescents (25), which is important for both athletic success and overall health. However, it is also crucial to consider the impact of this training on bone health and development to ensure safety during training (13). Previous studies have shown that plyometric training positively influences the growth and development of adolescents (14). The duration and intensity of the training play a crucial role in shaping the body and physiological systems (14). When comparing the training group to the CG, the efficacy of this specific training program was evident, with significant increases in GH (~12%), IGF-1 (~8%), and vitamin D levels. These findings suggest that plyometric training can be a valuable tool for promoting healthy growth and development.
Throughout adolescence, there is a notable increase in GH levels, which subsequently facilitates the secretion of insulin-like growth factor 1 (IGF-1) (26). This hormone plays a critical role in the development of bones, muscles, and ligaments, promoting healthy growth in these areas. Plyometric training, which involves explosive movements like jumping and hopping, is particularly beneficial during this developmental stage due to its alignment with heightened GH secretion (27). It enhances explosive power and muscle strength, and when done appropriately, the increased IGF-1 secretion stimulates healthy bone, muscle, and ligament development, reducing the risk of fractures and improving bone density (14).
Jumping ability is a critical physical skill, especially for young athletes in sports such as basketball, volleyball, high jump, and long jump, where it significantly impacts performance (28). However, good jumping ability is also essential for all adolescents. Extensive research has confirmed a significant and undeniable link between physical activity and improved quality of life (13). During the 12-week study, only one injury was reported, suggesting that it is safe to increase both the intensity and volume of loads for this particular population without incurring significant safety risks.
It is important to acknowledge that the DXA assessment used in this study has certain limitations due to its two-dimensional nature. It is possible that the implemented jumping intervention induced structural changes in areas not detectable by DXA. Additionally, the follow-up period in this study was relatively brief, so it would be beneficial for future research to incorporate measurements of bone geometry to better assess the impact of the intervention on bone structure and whether these changes persist over time.
5.1. Conclusions
The study found that plyometric training offers significant skeletal benefits to young participants without compromising their physical well-being. Improving strength, power, and body composition is crucial for establishing and maintaining physical wellness. Motor skill development is vital for achieving these goals in both children and adults. This type of training can also promote lifelong physical activity in boys who might otherwise lead a sedentary lifestyle. The study identified significant improvements in BMC across various sites, including an increase in trochanteric BMC. Incorporating plyometric training in high school PE programs can help reduce the future risk of hip fractures by improving bone density and strength. Raising awareness of its benefits on bone mass can encourage the inclusion of plyometric training in physical education curricula. Therefore, educational institutions should consider introducing plyometric training as part of their physical education programs.