1. Background
The therapeutic potential of exercise in treating and preventing neurological diseases is well-established. Exercise has been shown to have beneficial effects on clinical complications associated with neurodegenerative diseases (1, 2). These benefits may arise through the modulation of various brain-supporting mechanisms, including synaptogenesis, neurogenesis, metabolism regulation, and angiogenesis, all contributing to brain plasticity and maintenance (3). Furthermore, physical activities such as aerobic and resistance exercises have been found to delay the onset of neurodegenerative diseases and mitigate their harmful effects in older adults (4, 5).
Epilepsy is one of the most common neurological disorders (6, 7). Recent experimental and clinical studies suggest that regular moderate exercise is a helpful complementary therapy for managing epilepsy (8-13). Physical activity (PA) prior to epilepsy induction in animal models has been shown to reduce seizures (SE) and oxidative stress, while enhancing learning and memory through improved neurogenesis (14). Several studies indicate that exercise reduces the frequency and intensity of SEs by modulating various signaling pathways, including the remodeling of glial cells and regulation of glutamate/GABA receptors (6, 15, 16).
The endocannabinoid system is mediated by two G protein-coupled receptors: CB1 and CB2 (17). These receptors are expressed in different areas of the body, including the central nervous system and some peripheral tissues (18). CB1 and CB2 receptors are primarily localized in the brain and are found in several brain regions, such as the cortex, basal ganglia, and hippocampus (18, 19). The two main circulating endocannabinoid ligands for CB1 and CB2 receptors are 2-AG (2-arachidonoylglycerol) and anandamide (N-arachidonoylethanolamine; AEA). The activation of CB1 or CB2 receptors leads to various physiological effects on neural and glial cells, including gene transcription, neurotransmission, synaptic function, and cell motility (20).
The involvement of the endocannabinoid system in several brain functions—such as neural plasticity, learning and memory, neuronal development, and nociception—is well-documented (21-23). Activation of the endocannabinoid system regulates the function of neurotransmitter systems implicated in neurodegenerative diseases (24). The role of the endocannabinoid system in a range of neurodegenerative diseases, including multiple sclerosis, neuropathic pain, head trauma, ALS, and epilepsy, has been extensively studied (25, 26). Several studies have specifically focused on the involvement of the endocannabinoid system in epilepsy (27-29), leading to growing interest in targeting the cannabinoid system for therapeutic interventions in epilepsy (30-32). Understanding how exercise alters CB1 and CB2 receptor activity in the brain could help develop new therapeutic strategies involving receptor agonists or antagonists to prevent or treat epileptic SEs.
2. Objectives
Given the beneficial effects of exercise on individuals with epilepsy and its regulatory impact on the endocannabinoid system, we hypothesized that the endocannabinoid system plays a key role in mediating the positive effects of exercise on epilepsy. Thus, this study aimed to investigate the effect of PA on the modulation of CB1 and CB2 receptors in the brains of epileptic rats.
3. Methods
This study was conducted on 30 adults male Wistar rats, each weighing between 250 and 300 grams. All experiments were performed in accordance with the approved protocol of the Animal Ethics Committee of Iran University of Medical Sciences (IR.IUMS.REC.1400.938). The animals were housed under 12-hour light/dark cycles at a temperature of 23°C, with free access to water and standard food. After a few days of habituation, the animals were randomly divided into five groups, with six rats in each group.
The groups were as follows: (1) sham group: Animals received intraperitoneal (i.p.) injections of 0.9% saline on alternate days for four weeks; (2) SE group: Animals received pentylenetetrazol (PTZ) injections (35 mg/kg; i.p.) on alternate days for four weeks to induce SEs; (3) physical activity group: Animals were subjected to forced running on a treadmill for 30 minutes per day, 5 days a week, for 4 weeks; (4) PA and SE group: Animals received PTZ injections according to the SE group protocol and also underwent forced running on a treadmill using the same protocol as the PA group; (5) PA before SE group: Animals were forced to run for 30 minutes daily during weeks 1 to 4 without PTZ injections, and continued with the same protocol as the PA & SE group from weeks 5 to 8.
3.1. Exercise Protocol on a Treadmill
Animals underwent moderate-intensity exercise on a treadmill set at a 0° incline every other day for a total of 30 minutes per session. The running speed was progressively increased: 10 m/min for the first 5 minutes, 15 m/min for the next 5 minutes, 20 m/min for the following 5 minutes, and 25 m/min for the last 9 minutes. The first week was designated as a familiarization period for the treadmill exercise.
3.2. Immunohistochemistry
On the final day of the fourth week, 5 animals from each group were deeply anesthetized with chloroform and transcardially perfused with 250 mL saline followed by 400 ml of 4% paraformaldehyde (PFA). The brains were dissected and fixed in 4% PFA for 4 days. Coronal sections (8 μm thick) were obtained from paraffin-embedded brain tissue, corresponding to the somatosensory cortex (from 1.8 mm to 3.24 mm) and hippocampal CA1 and CA3 regions (from 3.80 mm to 4.1 mm posterior to the bregma). The sections were cleared in xylene and rehydrated through a graded alcohol series. After three washes with PBS, the sections were boiled in Tris-EDTA buffer at 95°C for 20 minutes. Once cooled to room temperature, the sections were washed with PBS and incubated in 1% BSA/Tris 0.1M (blocking solution) for 10 minutes. The sections were then incubated overnight at 4°C with primary antibodies, followed by a 2-hour incubation with a FITC-conjugated secondary antibody. Microscopic images of the cortex and hippocampal CA1 and CA3 regions were captured, and the number of reactive cells was counted using ImageJ software.
3.3. Statistical Analysis
Data were presented as mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Hippocampal and Cortical Distribution of CB1 Receptors
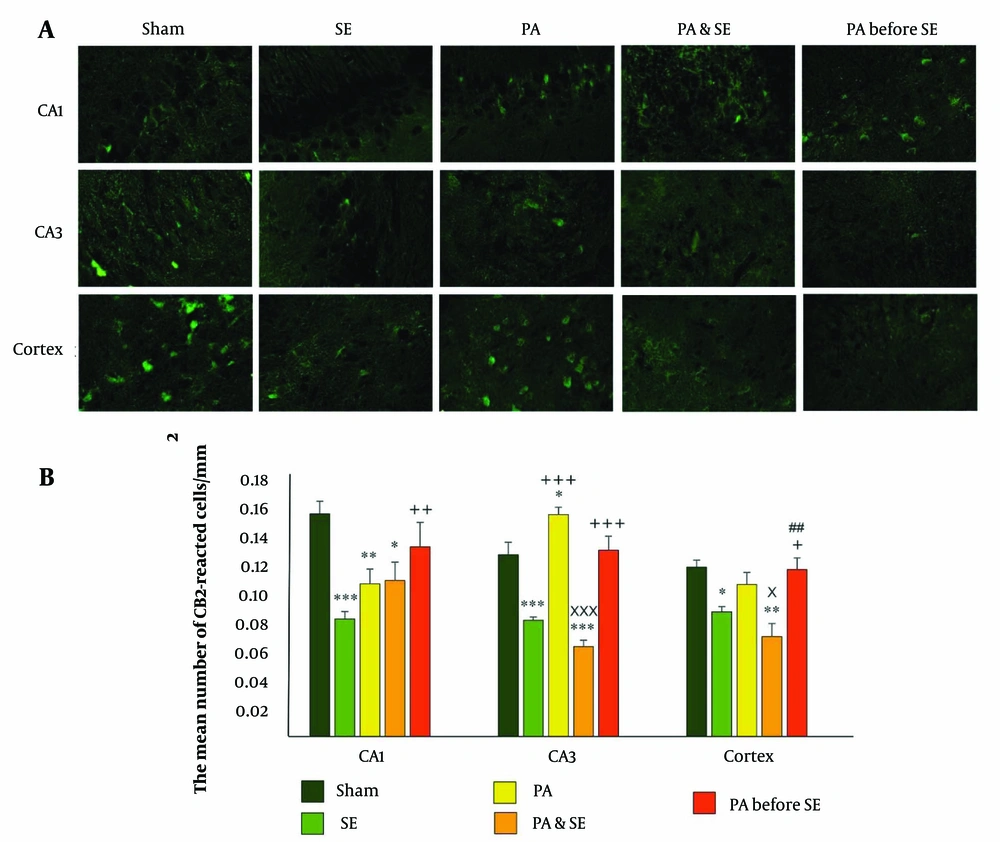
The results showed a significant upregulation of CB1 receptors in the CA1 region in both the PA group (0.12 ± 0.013, P = 0.03) and the PA before SE group (0.13 ± 0.017, P = 0.01) compared to the SE group (0.08 ± 0.005). Additionally, CB1 receptor levels were significantly downregulated in the SE group compared to the Sham group (0.14 ± 0.008, P = 0.01). No significant differences were observed between the Sham, PA, PA & SE, and PA before SE groups (Figure 1).
1 receptors distribution in the cortex, CA1 and CA3 hippocampal areas. (A), representative immunofluorescent photos of CB1 expression in the hippocampal and cortical areas; (B), the bar graph represents the mean number of reacted cells ± SEM (per mm2). *and** and *** represent respectively P < 0.05, P < 0.01 and P < 0.001, in comparison with the Sham group. +, ++ and +++ represent P < 0.05, P < 0.01 and P < 0.001, respectively compared to the SE group. x and xxx represent P < 0.05 and P < 0.001 respectively compared to the PA group. ## represent P < 0.01 in comparison with the PA & SE group.
In the CA3 region, CB1 receptor expression significantly decreased in the SE group (0.081 ± 0.002, P = 0.0001) and the PA & SE group (0.063 ± 0.004, P = 0.0001), while it increased in the PA group (0.15 ± 0.005, P = 0.025) compared to the Sham group (0.12 ± 0.008) (Figure 1). Moreover, CB1 receptors were significantly upregulated in the PA & SE and PA before SE groups (0.13 ± 0.009) compared to the SE group (P = 0.0001). The PA before SE group also showed increased CB1 receptor expression compared to the PA & SE group (P = 0.0001).
In the cortical area, significant downregulation of CB1 receptors was observed in the SE group (0.088 ± 0.003) and the PA & SE group (0.07 ± 0.009) compared to the Sham group (P = 0.023 and P = 0.002, respectively). In the PA before SE group, CB1 receptor expression was significantly higher compared to the SE and PA & SE groups (P = 0.035 and P = 0.004, respectively) (Figure 1).
4.2. Hippocampal and Cortical Distribution of CB2
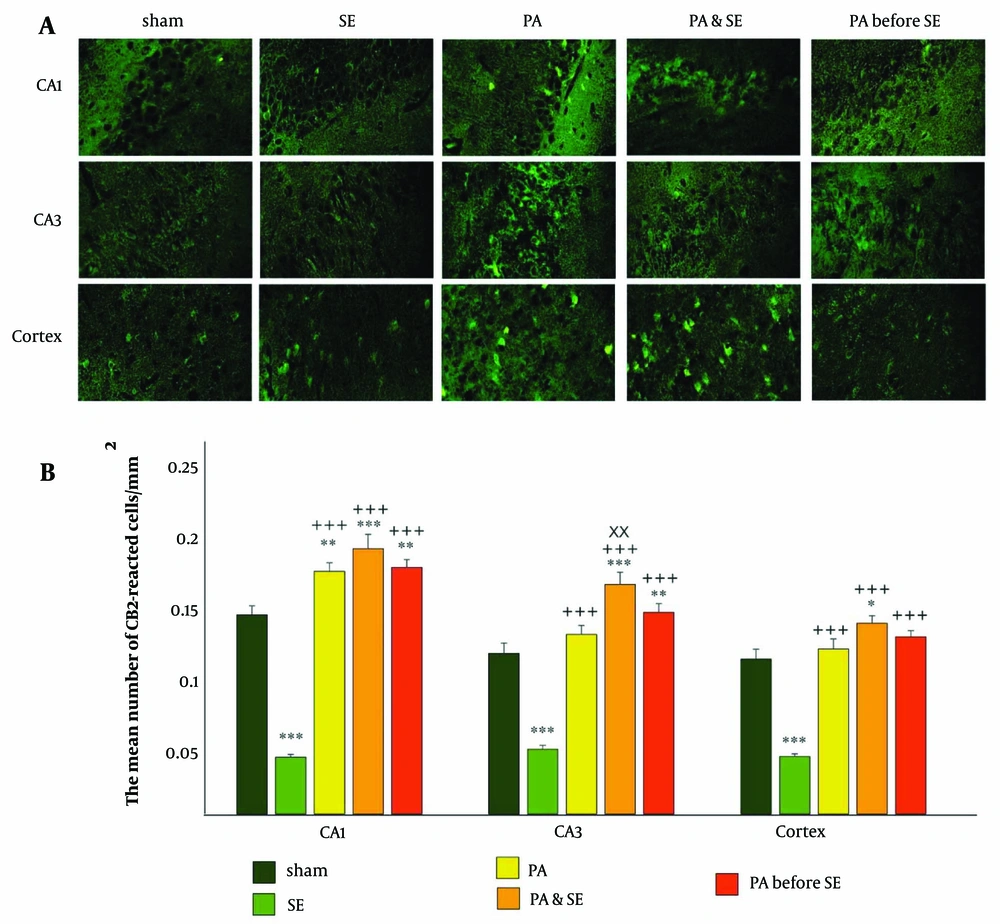
In the CA1 region, a significant increase in CB2 receptor expression was observed in the PA, PA & SE, and PA before SE groups compared to the Sham and SE groups (Figure 2 P = 0.007 and P = 0.001, respectively). CB2 receptor levels were notably decreased in the SE group compared to the Sham group (P = 0.001). No significant differences were found between the PA, PA & SE, and PA before SE groups. The mean ± SEM values were 0.14 ± 0.006, 0.04 ± 0.002, 0.17 ± 0.006, 0.19 ± 0.01, and 0.17 ± 0.005 for the Sham, SE, PA, PA & SE, and PA before SE groups, respectively.
Distribution of CB2 receptor in the cortex, CA1 and CA3 hippocampal areas. (A), representative immunofluorescent photos of CB1 expression in the hippocampal and cortical areas; (B), the bar graph represents the mean number of reacted cells ± SEM (per mm2). *, ** and *** represents respectively P < 0.05, P < 0.01 and P < 0.001, in comparison with the Sham group. +++ represents P < 0.001 in comparison with the SE group. xx represents P < 0.01 in comparison with the PA group.
In the CA3 region, CB2 receptor expression significantly increased in the PA & SE and PA before SE groups, while it decreased in the SE group compared to the Sham group (Figure 2 P = 0.015 and P < 0.001, respectively). Additionally, CB2 expression was significantly elevated in the PA, PA & SE, and PA before SE groups compared to the SE group (P = 0.001). Notably, CB2 expression was higher in the PA & SE group compared to the PA group (P = 0.014). The mean ± S.E.M values were 0.11 ± 0.007, 0.047 ± 0.002, 0.13 ± 0.006, 0.16 ± 0.008, and 0.14 ± 0.006 for the Sham, SE, PA, PA & SE, and PA before SE groups, respectively.
In the cortical area, CB2 receptor distribution significantly increased in the PA & SE group and decreased in the SE group compared to the Sham group (Figure 2 P = 0.033 and P = 0.0001, respectively). CB2 receptor distribution was significantly elevated in the PA, PA & SE, and PA before SE groups compared to the SE group (P = 0.0001). No significant differences were noted between the PA, PA & SE, and PA before SE groups. The mean ± SEM values were 0.11 ± 0.007, 0.041 ± 0.002, 0.12 ± 0.007, 0.13 ± 0.005, and 0.12 ± 0.004 for the Sham, SE, PA, PA & SE, and PA before SE groups, respectively.
5. Discussion
In this study, we evaluated the effect of exercise before and during epileptic conditions on the hippocampal and cortical distribution of CB1 and CB2 cannabinoid receptors.
It is well known that an imbalance between excitatory (glutamatergic) and inhibitory (GABAergic) signals is the primary trigger of epileptic SEs (33, 34). The endocannabinoid system plays a crucial role in activity-dependent retrograde synaptic neurotransmission, regulating both glutamatergic and GABAergic neurotransmission via CB1 receptors. Studies have shown that endocannabinoids released from postsynaptic neurons activate presynaptic CB1 receptors on axonal terminals, inhibiting neurotransmitter release. The activation of CB1 receptors on excitatory glutamatergic axonal terminals has been suggested to have anti-epileptic effects (35). Additionally, cannabinoids have been shown to reduce neuronal excitability and SE activity by activating muscarinic receptors, which are key regulators of neural excitability. Specifically, CB1 receptors exhibit inhibitory interactions with muscarinic receptors on cholinergic neurons in the hippocampus of rodents. Seizures induced by the muscarinic agonist pilocarpine, due to M1 receptor activation, can be modulated by CB1 receptors (36).
Previous research has indicated that the genetic ablation or acute pharmacological blockade of CB1 or CB2 receptors by specific antagonists lowers the SE threshold and facilitates epileptogenesis (37, 38). A significant reduction in the expression of CB1 receptors and DAGL-α (a 2-AG synthesizing enzyme) has been identified in postmortem hippocampal samples from epileptic patients (39). In alignment with these findings, our results revealed a marked reduction of CB1 and CB2 receptors in the CA1, CA3, and cortex of epileptic animals compared to healthy subjects.
Moderate, regular exercise has been suggested as a complementary therapy for epilepsy (40-42). Exercise has been shown to minimize the frequency and intensity of SEs, as well as reduce brain cell loss or neuronal damage following SE attacks (9, 12). The endocannabinoid system has been recognized as a key mechanism involved in the beneficial effects of exercise on neurological disorders (43).
Our study demonstrated that the expression of CB1 and CB2 receptors was significantly reduced under epileptic conditions. In contrast, exercise increased CB1 and CB2 receptor expression in the hippocampus and cortex of non-epileptic animals. Furthermore, exercise ameliorated the deficit in hippocampal and cortical CB2 receptors in epileptic animals. These findings highlight the potential role of exercise in modulating the endocannabinoid system, thereby contributing to the anti-epileptic effects of PA.
Previous studies have reported contradictory findings regarding CB1 receptor distribution in exercised animals. For instance, unlike our results, Gomes da Silva et al. observed a reduction in hippocampal CB1 receptors in treadmill-exercised adolescent male rats, while no significant changes were found in cortical CB1 expression following exercise (44). On the other hand, Hill et al. showed that running wheel exercise increased the density of CB1 receptors and the tissue content of the endocannabinoid anandamide in the hippocampal dentate gyrus but not in the prefrontal cortex. They also suggested that endocannabinoid signaling in the hippocampus mediated exercise-induced increases in cell proliferation and plasticity (45). This discrepancy may be explained by the findings of Magloczky et al. Their molecular assays on both epileptic mice and human hippocampi revealed that in chronic epilepsy, although the density of CB1 receptors was reduced on glutamatergic axons, there was a notable increase in CB1 receptor-expressing GABAergic fibers sprouting in the dentate molecular layer. Additionally, CB1 receptor levels on these GABAergic axons increased. Thus, the overall level of CB1 receptors was preserved in both the CA1 and dentate gyrus regions, suggesting that this GABAergic inhibitory system may correlate with the severity of epilepsy (35).
In addition to CB1 receptors, CB2 receptors have been shown to mediate neural plasticity in the hippocampus (46). Activation of CB2 receptors has been reported to trigger action potentials, leading to prolonged membrane hyperpolarization in CA3 and CA2 pyramidal cells. These findings highlight the expression of CB2 receptors in the hippocampus and emphasize their critical role in neuronal transmission (47).
Moreover, Rowley et al. found that CB1 receptor knockout mice did not exhibit an epileptic phenotype, but co-knockout of both CB1 and CB2 receptors resulted in epilepsy (48). Shapiro et al. reported that administering a CB2 receptor antagonist increased SE susceptibility in a chemical model of epilepsy (49), while administration of a CB2 agonist alleviated kainic acid-induced status epilepticus in rats (50). In line with these findings, our study demonstrated that CB2 receptor expression was reduced in epileptic animals, but exercise restored CB2 receptor levels in the hippocampus and cortex.
As a potential mechanism, recent investigations suggest that CB2 receptor activation reduces excitatory synaptic transmission in the brain. Specifically, it has been found that CB2 receptor activation induces hyperpolarization in CA1 and CA3 hippocampal pyramidal cells and suppresses SE activity (51). Stumpf et al. also indicated that CB2 receptors mediate retrograde inhibition in neurons of layer 2/3 of the somatosensory cortex, suggesting a role for CB2 receptors in modulating neuronal excitability (19). These findings underscore the stabilizing function of CB2 receptors in the neuronal system and suggest their modulatory effects on SE susceptibility (38).
Our data further revealed that when exercise was initiated simultaneously with SE development, CB2 receptors were upregulated, but not CB1 receptors. However, prioritizing physical exercise before the onset of SEs led to an increase in both CB1 and CB2 receptors in the cortex and hippocampus.
This suggests that CB2 receptors played a critical role in ameliorating endocannabinoid system dysfunction in epileptic rats, and animals that underwent exercise before SE induction were more resistant to the imbalance in the endocannabinoid system caused by SEs. Given previous studies highlighting the stabilizing function of CB2 receptors in the neuronal system (as discussed earlier), these findings emphasize the potential of targeting CB2 receptors as a novel therapeutic strategy for preventing or treating epileptic SEs.
5.1. Limitation
It would have been advantageous to measure cannabinoid protein levels using the western blot technique to more accurately assess protein changes and their correlation with behavioral alterations.
5.2. Conclusions
Our findings indicated that exercise modulated endocannabinoid receptors in the hippocampus and cortex of epileptic animals, suggesting that the endocannabinoid system may play a critical role in mediating the beneficial effects of exercise on epilepsy.