1. Background
Performing an unaccustomed exercise causes symptoms characterized by muscle weakness, stiffness, soreness and swelling (1). It is known that eccentric exercise (ECC) produces these symptoms greater than concentric exercise (2), and the symptoms are often used as markers of muscle damage (1, 3). Other studies of exercise-induced muscle injury have reported increase in plasma level of intracellular muscle proteins (i.e. creatine kinase (CK), myoglobin, lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) (4)) as markers of injury. Eccentric exercise induces ultra-structural muscle damage within sarcomere? Leading to membrane damage and failure of excitation contraction coupling pathway (5, 6). It was suggested that exercise induced muscle damage was attenuated with heat shock proteins (HSPs) (7). Heat preconditioning induces (HSPs), which protect cells by promoting synthesis of and controlling the degradation of muscle proteins (5, 8). Heat induced HSP 72 protects skeletal muscle against impairments of excitation contraction coupling (9). It has been reported that an elevation of HSP expression peaked between 24 and 48 hours following heat where the core temperature of rats was maintained around 41°C for 30 minutes (10). A study (11) documented 24 hours prior thermal preconditioning (immersion in water at 41.5 + 0.5°C for 20 minutes) has attenuated exercise induced muscle injury in mice. It was reported previously (12) that heat preconditioning using microwave diathermy (150 Watts, 20 minutes) increased the temperature of vastus lateralis to over 40°C and after 1 day resulted in the release of several HSPs (HSP 90, HSP 72, HSP 27) in human skeletal muscles. One day prior heat preconditioning using microwave diathermy (150 watts, 20 minutes) against exercise induced muscle damage was found to be significantly effective in the faster recovery of maximal voluntary isometric contraction strength (MVC), associated with a smaller decrease in range of motion (ROM) with no effect on the upper arm circumference increase and CK activity post exercise (13, 14). Soreness on extension was also found to be reduced with microwave diathermy treatment (13). At first, it was found that 2 days prior 10% ECC that does not result in any changes in muscle damage markers, was effective in inducing faster recovery of MVC, ROM and reduced muscle soreness following submaximal eccentric exercise (15), suggesting that the light eccentric exercise preconditioned the muscle against a damaging exercise bout. Other studies (16, 17) extended the previous findings and reported the prophylactic effects of 10 % ECC against Max-ECC. These studies resulted that 10% ECC has induced faster recovery of MVC, ROM, soreness, upper arm circumference and CK activity after Max ECC performed 2 days (16) or 7 days (17) later.
2. Objectives
Previous researches have already mentioned that both 10% ECC and heat using microwave diathermy precondition the muscles for subsequent damaging exercise bout, but no study has focused on the comparison of their effects. Therefore, purpose of the present study was to compare their preconditioning effect and to establish which one of them would be more beneficial in reducing muscle damage.
3. Patients and Methods
3.1. Subjects
Thirty six sedentary collegiate males (age 19-26 years) from Jamia Millia Islamia University, India, participated in the study. Ethical approval was granted by Institutional Human Ethical Committee and the subjects were given written informed consent. The subjects were explained the purpose and methodology and possible risks of the study. None of the subjects were involved in regular resistance training for at least 12 months prior to this investigation and all of them were free from any musculoskeletal disorder of upper extremities. The sample size was estimated using data of changes in maximal isometric strength from a previous study (14) in which a similar heat preconditioning using microwave was performed and the subjects from same population were used and eighteen subjects (n = 18) per group were shown to be necessary based on the effect size of (d) 1.27, alpha level of 0.05 and a power (1-β) of 0.95.
3.2. Procedure and Protocol
A comparative, parallel group, prospective study design, random sampling was done for allocation of subjects into two experimental groups (n = 18 each group). Both the experimental groups performed Max-ECC of the elbow flexors with their non-dominant arm. 10% ECC group performed a bout of light load eccentric exercise of the elbow flexors of the non-dominant arm 2 days prior to maximal eccentric exercise (Max-ECC). Microwave diathermy (MWD) group received microwave diathermy treatment 1 day prior to Max-ECC. In both the groups, MVC, ROM, upper arm circumference, soreness were measured immediately before and 24-72 hours following the Max-ECC. Whereas serum CK and LDH activity were assessed immediately before and 24-48 hours following the Max-ECC. All of the measures were taken from the exercised arm and blood samples were taken from the contralateral arm.
MVC: Subjects were positioned supine on the couch. The non-dominant arm was positioned with the shoulder abducted 30°, the elbow flexed 90° and forearm supinated (18) and the strength was measured with the help of a strain gauge. The subjects were asked to perform two maximal isometric contractions for 3 seconds each with 30 second rest in between the efforts. The average of the two MVC values was used for the determination of the exercise load (15).
Max-ECC: Dumbbell weighing each of individual’s MVC strength at 90° elbow flexion was used. For the exercise of the elbow flexors, subjects were seated on a standard arm curl bench with the shoulder flexed at 45° and the forearm in a supinated position. The Max-ECC protocol described below was used in the previous study (19). Subjects were instructed to lower the dumbbell from an elbow flexed (50°) to elbow extended position (170°) in 4-5 seconds keeping the velocity as constant as possible (30°/second). After each eccentric contraction, the examiner removed the load and the arm was returned to the starting position without load. The movement was repeated every 45 seconds for 30 repetitions. 10% ECC: Dumbbell set at 10% of MVC strength at 90º elbow flexion. The dumbbell weight was adjusted by sticking small lead bars (100 g each) to the dumbbell with tape. The 10% ECC consisted of five sets of six eccentric contractions, in which the subjects were instructed to lower the dumbbell from elbow flexed (90°) to an elbow fully extended position (0°) in 3 seconds. The dumbbell was removed by the therapist at the extended position and the arm was returned to the start position without load (15, 19). The interval between contractions was 10 seconds and a 2 minute rest between the sets was given (16). Heat preconditioning: The non-dominant upper arm was exposed to heat preconditioning using microwave diathermy set at 150 watts for 20 minutes (13, 14). Subjects in the sitting position with the treatment arm relaxed and stretched on a bench placed in front of the body (13).
3.3. Dependent Variables
MVC: As explained previously, MVC was measured at 90º elbow flexion using strain gauge. Two measurements for maximal effort were taken and the average of the two values was used for further analysis (15).
ROM: ROM of the elbow joint was determined as the difference between the elbow joint angles of maximal flexion (FANG) and extension (EANG). The FANG was measured at maximal elbow flexion, and the EANG was measured at maximal elbow extension. A Universal plastic goniometer was used for the measurement. To ensure that measurements were taken from the same point each time, a semi-permanent ink pen was used to mark a point over the proximal apex of the deltoid, the axis of rotation of the elbow, the styloid process and dorsal tubercle of the radius (13). Upper arm circumference: Upper arm circumference was measured using a Gulik constant tension tape. Measurements were taken at 3, 5, 7, 9 and 11 cm proximal from elbow crease of cubital fossa and the mean value of the five sites was used for further analysis (15). Blood markers: Approximately 2 mL of venous blood was drawn from an anticubital vein of the dominant arm (non-exercised arm) by a standard venepuncture technique using a disposable needle. The blood was then centrifuged to separate the serum. The serum CK and LDH activity was monitored by a spectrophotometer at 340 nm set wavelength using a CK KIT and LDH KIT respectively. Muscle soreness: Subjects were asked to indicate their pain level on a 10 cm visual analogue scale (VAS) while their elbow flexors were being extended (13).
3.4. Statistical Analysis
Data were assessed by Shapiro-Wilk test for the normality of the distribution scores. The demographic characteristics and the baseline dependent variables prior to Max-ECC were compared between groups by an independent t-test. Changes in the dependent variables over time following Max-ECC were compared between groups by a mixed model ANOVA. Also, Bonferroni test was employed as post hoc analysis to locate the time points having significant difference. Significance level was set at P < 0.05. The data were presented as mean (Standard deviation).
4. Results
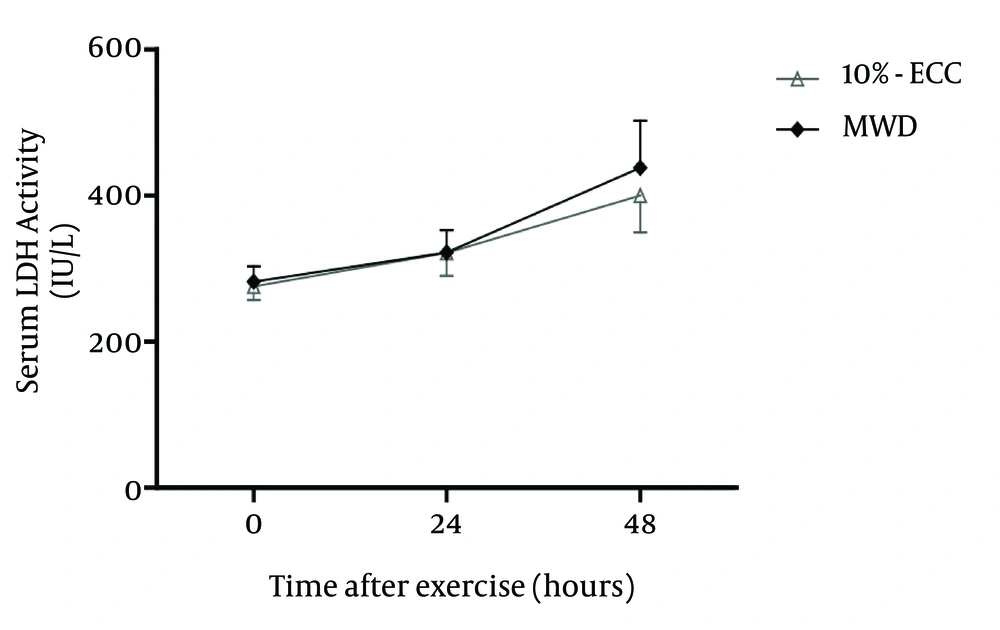
Shapiro-Wilk test indicated that dependent variables in both groups were normally distributed. No significant difference for the demographic characteristics and baseline dependent variables existed between groups (Table 1). No significant relation between group and time was found in case of MVC, ROM, upper arm circumference, soreness on extension and CK activity for changes from 0 to 72 hours post Max ECC (Table 2). However in case of LDH activity, a significant relation between group and time was found for changes from 0 to 48 hours post Max-ECC (Table 2 and Figure 1).
| Variable | 10% Eccentric Exercise Group | Microwave Diathermy Group | P Valuec |
|---|---|---|---|
| Age, y | 22.00 ± 2.30 | 22.22 ± 2.10 | 0.8 |
| Height, m | 1.70 ± 0.05 | 1.70 ± 0.01 | 0.8 |
| Weight, kg | 63.66 ± 8.7 | 63.55 ± 2.4 | 1 |
| BMI, kg/m2 | 21.93 ± 2.87 | 21.95 ± 3.20 | 1 |
| MVC, kg | 13.26 ± 1.14 | 12.90 ± 2.07 | 0.5 |
| Range of motion, degrees | 128.28 ± 3.41 | 125.83 ± 4.29 | 0.07 |
| Upper arm circumference, cm | 24.18 ± 1.98 | 24.79 ± 2.96 | 0.5 |
| Serum creatine kinase, IU/L | 53.55 ± 13.49 | 60.33 ± 14.72 | 0.2 |
| Serum lactate dehydrogenase, IU/L | 275.76 ± 18.45 | 282.44 ± 20.77 | 0.3 |
aAbbreviations; BMI, body mass building; MVC, maximal voluntary isometric contraction strength
bData are presented as Mean ± SD.
cIndependent t-test.
| Source | P Value |
|---|---|
| Maximal voluntary isometric contraction strength, kg | |
| Group (G) | 0.5 |
| Time (T) | < 0.001 |
| Interaction (G×T) | 0.08 |
| Range of motion, degrees | |
| Group (G) | 1 |
| Time (T) | < 0.001 |
| Interaction (G×T) | 0.06 |
| Upper arm circumference, cm | |
| Group (G) | 0.5 |
| Time (T) | < 0.001 |
| Interaction (G×T) | 0.4 |
| Soreness on extension, cm | |
| Group (G) | 0.4 |
| Time (T) | < 0.001 |
| Interaction (G×T) | 0.7 |
| Creatine kinase activity, IU/L | |
| Group (G) | 0.9 |
| Time (T) | < 0.001 |
| Interaction (G×T) | 0.08 |
| Lactate dehydrogenase activity, IU/L | |
| Group (G) | 0.1 |
| Time (T) | < 0.001 |
| Interaction (G×T) | 0.04 |
5. Discussion
The results of the present study demonstrated that the preconditioning effects of light load eccentric exercise and heat using microwave diathermy treatment were similar on the markers of muscle damage (MVC, ROM, upper arm circumference, soreness, CK activity) except for the changes on LDH activity; following Max-ECC (Table 2). The present study is the first study comparing these 2 experimental groups as well as highlighting their effects on LDH activity. In the present study, MVC strength decreased up to 48 hours in a similar manner in both groups with a peak reduction of 34.1% in 10% ECC group and 29.4% in MWD group (Table 3). Research has demonstrated that calcium release from the sarcoplasmic reticulum is impaired in injured muscles. Eccentric contractions have also been shown to alter the structure of T-tubules. Both of these changes would be responsible for the impairment of excitation-contraction coupling (4). Moderately intensive eccentric exercise results in strength losses of 30-50%, while intense protocols reduce strength by 50-70%, with recovery times of 7-10 days, extending to several weeks (20). ROM decreased up to 48 hours in a similar manner in both groups, with a peak reduction of 16.34° in 10% ECC group and 12.94° in MWD group (Table 3). It has been suggested that an increase in the number of contracted fiber segments related to either an increase in resting cytosol calcium levels or ultrastructural damage may be responsible for ROM reduction (21). Significant increase in the muscle soreness was observed in both groups with a peak value at 48 hours post exercise (Table 3) which is in line with the other previous studies (13, 15-17). The primary mechanisms underlying the delayed onset muscle soreness (DOMS) sensations include swelling and subsequent increased pressure within the muscle that activates resident mechanoreceptors (nociceptors). The chemical changes responsible for DOMS include increased histamine, bradykinins and prostaglandins that follow the infiltration of inflammatory cells. These substances activate the polymodal nociceptors that are sensitive to chemical signals (20). Peak value of soreness on extension at 48 hours post exercise for 10% ECC is 3 cm, which was also reported previously as 2.5 cm (15) and for MWD it was also 3cm (Table 3), as similarly reported previously (13). After 48 hours, both the groups recovered MVC with similar rates (10% ECC = 11%, MWD = 11%) as well as a similar amount of increase in the ROM (10 % ECC = 4°, MWD = 6°), statistically not significant and recovery of soreness started in both groups. Both the groups showed increment in the upper arm circumference up to 72 hours following Max-ECC in a similar manner. Magnetic resonance imaging has demonstrated intracellular edema in muscle tissue after eccentric exercise (22). The amount of increase in upper arm circumference from pre exercise value to 72 hours post exercise in 10% ECC was 8 mm which is quite near to previous studies (16) reporting approximately 5 mm and in MWD the increase was 7 mm which is quite near to previous studies (14) reporting approximately 10 mm.
| Time, h | Group | MVC, kg | ROM, degrees | UAC, cm | SOE, cm | CK, IU/L | LDH, IU/L |
|---|---|---|---|---|---|---|---|
| 0 | 10% ECC | 13.26 ± 1.14 | 128.28 ± 3.41 | 24.18 ± 1.98 | 0 | 53.55 ± 13.49 | 275.76 ± 18.45 |
| 0 | MWD | 12.90 ± 2.07 | 125.83 ± 4.29 | 24.79 ± 2.96 | 0 | 60.33 ± 14.72 | 282.44 ± 20.77 |
| 24 | 10% ECC | 9.49 ± 1.51 | 118.06 ± 5.51 | 24.70 ± 2.00 | 2.17 ± 0.62 | 100.80 ± 10.17 | 321.61 ± 31.38 |
| 24 | MWD | 10.21 ± 1.77 | 118.06 ± 3.72 | 25.30 ± 2.87 | 1.94 ± 0.73 | 96.77 ± 18.23 | 322.27 ± 30.45 |
| 48 | 10% ECC | 8.71 ± 1.52 | 111.94 ± 5.69 | 25.01 ± 2.10 | 3.00 ± 0.49 | 128.85 ± 14.98 | 400.06 ± 50.35 |
| 48 | MWD | 9.06 ± 1.97 | 112.89 ± 3.01 | 25.52 ± 2.90 | 3.00 ± 0.59 | 128.27 ± 20.39 | 438.44 ± 64.19 |
| 72 | 10% ECC | 9.69 ± 1.38 | 116.39 ± 6.33 | 24.96 ± 2.02 | 1.89 ± 0.58 | – | – |
| 72 | MWD | 10.43 ± 1.56 | 118.00 ± 3.38 | 25.46 ± 2.84 | 1.72 ± 0.67 | – | – |
| 0 vs. 24 post hoc | 10% ECC | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| 0 vs. 24 post hoc | MWD | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | <0.001 |
| 24 vs. 48 post hoc | 10% ECC | 0.3 | < 0.001 | < 0.001 | 0.002 | < 0.001 | < 0.001 |
| 24 vs. 48 post hoc | MWD | 0.049 | < 0.001 | 0.02 | < 0.001 | < 0.001 | < 0.001 |
| 48 vs. 72 post hoc | 10% ECC | 0.004 | 0.001 | 1.0 | <0.001 | - | - |
| 48 vs. 72 post hoc | MWD | < 0.001 | < 0.001 | 1.0 | < 0.001 | - | - |
aAbbreviations: CK, creatine kinase activity; LDH, lactate dehydrogenase activity; MVC, maximal voluntary isometric contraction strength; ROM, range of motion; SOE, Soreness on extension; UAC, upper arm circumference.
bData are presented as Mean ± SD.
Both the groups showed significant serum CK and LDH elevations up to 48 hours post exercise, consistent with a previous study (23). When the exercise intensity is within the normal range of metabolism, the muscle tissue is exercised without marked changes in membrane permeability. However, when the exercise intensity exceeds this permissible range, the membrane permeability temporarily changes, resulting in CK release from the active muscle. The boundary of this permissible range is its break point (24). From 0 to 48 hours, for CK activity, 10% ECC showed an increase of 75.3 IU/L and MWD showed an increase of 67.94 IU/L (Table 3), (statistically not significant). For LDH activity, the amount of increase in 10% ECC group and in MWD group was 124.3 IU/L and 156 IU/L (Table 3 and Figure 1), respectively which was found to be statistically significant (Table 2). One limitation of the study was that analysis of LDH activity was done only up to 48 hours so further analysis is recommended. The present study concluded that 2 days prior light load eccentric exercise or 1 day prior heat using microwave diathermy had similar effects on muscle damage markers after Max-ECC. Therefore both of them can be used interchangeably as a preventive measure against muscle damage in clinical settings depending upon the availability of the equipment, therapist’s skill or knowledge and client’s preference. However with time, their effects on LDH activity were found to be different. Further studies are therefore recommended to establish a more detailed analysis of the effects on changes in LDH activity by comparison up to several days post exercise.