1. Background
Participating in a regular exercise program is necessary to prevent the undeniable diseases and improve one’s quality of life. However, induced mechanical stress (metabolic) with eccentric contractions, including physical activity like running downhill, may cause overproduction of free radicals and non-enzymatic depletion of endogenous anti-oxidants, increased oxidative stress (primary and secondary) and damage to biological macromolecules, including nucleic acids, proteins and lipids in the human body (1-3). Therefore, scientists are constantly seeking ways to improve exercise capacity through the addition of health promotion strategies and anti-oxidants, to prevent the accumulation of oxidative damage indices such as malondialdehyde (MDA), protein carbonyl and 8-hydroxy-2-deoxy guanosine (8-OH-dG) (3, 4). In this regard, the results of some studies have suggested that intake of caffeine or 1.3.7 Tri methyl xanthine as a lipid-soluble alkaloid and a popular ergogenic supplement (available in many drinks such as tea and coffee) may neutralize free radicals to prevent the occurrence of pressure and elevated markers of oxidative damage. The results of the study by Corsetti et al. (2010) suggest that caffeine intake in different amounts (5/0 and 30 mg/day), replenishing reserves of glutathione (an endogenous, non-enzymatic anti-oxidant), decrease the levels of beta-amyloid (5). Mukhopadhyay et al. (2003) reported that short-term supplementation of caffeine (20 mg/kg body weight for 30 days) decrease MDA levels (6). In addition, Beck et al. (2006) reported that high doses of caffeine (150 to 200 mg/kg body weight) in damaged mice cerebral cortex causes lipid peroxidation (LPO) and oxidative stress (7). However, there is limited research related to the combined effect of caffeine on exercise reaction and on oxidative markers. Jafari et al. (2012) suggested that acute consumption of caffeine has many effects on the body at 4 mg serum anti-oxidant capacity, at 5 and 30 min after running 10 km (8). Jafari et al. (2011) also demonstrated that acute supplementation of caffeine (5 mg/kg of body weight) did not increase the levels of serum MDA after cycling for 30 min in order to avoid 75% peak aerobic power (9). Therefore, there is limited data and lack of systematic studies about the effects of acute caffeine intake on oxidative damage marker response during sporting activity.
2. Objectives
This study aims to determine the acute effect of caffeine (5 mg/kg body weight) on the response of some oxidative stress indicator [(total anti-oxidation capacity (TAC), MDA and serum total thiol molecules (TTM) and its DNA damage (8-hydroxy-2-deoxy guanosine (8-OH-dG) levels)] in non-athletic males.
3. Patients and Methods
The research design is in the form of a double-blind, quasi-experimental study, with repeated measurements conducted on 20 non-athlete males who were divided into two randomized, non-homogeneous groups: placebo (5 mg/kg of body weight dextrose) and caffeine capsule supplementation (5 mg/kg of body weight). The study was approved in Islamic Azad University of Jiroft Branch. Individual characteristics are presented in Table 1. The sample size and dose were based on previous studies. Ten patients were included in each group. The first blood samples were collected to evaluate antioxidant capacity, MDA concentrations, TTM and DNA damage 10 days before the vein elbow crutch caffeine supplementation. The overall aerobic power of the subjects was measured a week before measurement of the test beam. A week later (washout phase), the patients used a daily dose of caffeine (5 mg/kg of body weight) and dextrose (5 mg/kg of the conjectural body weight) in the supplementation and placebo groups, respectively, for 14 days. A second blood sample was collected 30 min before exercise and sporting activity. Each of the patients ran on a treadmill incline of 10° downhill for 30 min at an intensity of 65% maximal oxygen consumption. Immediately after blood sampling, the patients performed a third exercise test. The fourth blood sample was collected 24 h after the exercise test. During the project, patients were asked to complete a daily 24-h total diet recall questionnaire. In addition, they were asked not to participate in sports outside the study and avoid taking any drugs that were part of their treatment plan. TAC was measured using FRAP techniques, MDA was measured by the thiobarbituric acid method and TTM with Hu and DNA damage was evaluated using ELISA. The 8-OH-dG kit used an anti-mouse IgG-coated plate and a tracer consisting of an 8-OH-dG–enzyme conjugate and an 8-OH-dG antibody that distinguishes both free 8-OH-dG and DNA-incorporated 8-OH-dG. A complete blood count (CBC), especially the number of leukocytes, was performed using the H-1. Research data also confirmed the normality through the Kolmogorov-Smirnov Test. Data analysis was done for measuring mean and standard deviation using repeated ANOVA and the post hoc Bonferroni independent tests by SPSS software version 17 at a significance level of P = 0.05.
| Case Study Index/Group | Mean | Standard Deviation of Error | Standard Deviation |
|---|---|---|---|
| Age, y | |||
| Caffeine | 25.66 | 0.799 | 2.39 |
| Placebo | 25.22 | 0.571 | 1.71 |
| Weight, kg | |||
| Caffeine | 70.44 | 3.29 | 9.87 |
| Placebo | 71.05 | 1.95 | 5.86 |
| Height, cm | |||
| Caffeine | 175.28 | 2.01 | 6.04 |
| Placebo | 178.79 | 2.09 | 6.29 |
| Body mass index, kg/m2 | |||
| Caffeine | 22.74 | 0.741 | 2.22 |
| placebo | 21.80 | 0.528 | 1.56 |
| Fat Percentage, % | |||
| Caffeine | 13.665 | 0.784 | 2.35 |
| Placebo | 73.76 | 0.566 | 1.70 |
| Oxygen intake during exercise, mL/kg/min | |||
| Caffeine | 50.00 | 2.162 | 6.48 |
| Placebo | 49.88 | 1.12 | 3.38 |
4. Results
In the present study, we have shown that short-term supplementation of caffeine was correlated with significant changes in basal levels of total antioxidant capacity (TAC), serum malondialdehyde (MDA) and total thiol molecules (TTM) and did not induce 8-OH-dG index (P < 0.05). The results of repeated ANOVA suggest that running downhill causes significant changes of all parameters (TAC, TTM, LPO and 8OH-dG) measured in this study (Table 2).
| Parameters/Source of Change | Sum Square of Deviations From the Mean | df | Mean Square Deviations From the Mean | F | P Value |
|---|---|---|---|---|---|
| TAC, µM | |||||
| The effect of the measurement process | 2458.188 | 3 | 819.39 | 2.89 | 0.043 |
| Effects of group differences | 1571.14 | 1 | 1571.14 | 1.32 | 0.264 |
| The effect of group Steps differences measurement | 9876.80 | 3 | 3292.26 | 11.63 | P < 0.001 |
| TTM, nmol/mg protein | |||||
| The effect of the measurement process | 0.082 | 3 | 0.072 | 9.00 | P < 0.001 |
| Effects of group differences | 0.010 | 1 | 0.010 | 1.208 | 0.286 |
| Due to a difference in measured steps | 0.004 | 3 | 0.001 | 0.392 | 0.759 |
| LPO, µM | |||||
| The effect of the measurement process | 798.628 | 3 | 266.209 | 24.56 | P < 0.001 |
| Effects of group differences | 811.22 | 1 | 811.22 | 1.494 | 0.237 |
| The effect of group differences and measurement steps | 378.71 | 3 | 126.23 | 11.65 | P < 0.001 |
| 8OH-dG, pg/mL | |||||
| The effect of the measurement process | 48.935 | 3 | 16311.88 | 31.46 | P < 0.001 |
| Effects of group differences | 72965.61 | 1 | 72965.61 | 3.004 | 0.100 |
| The effect of group differences and measurement steps | 4124.36 | 3 | 1374.78 | 2.65 | 0.058 |
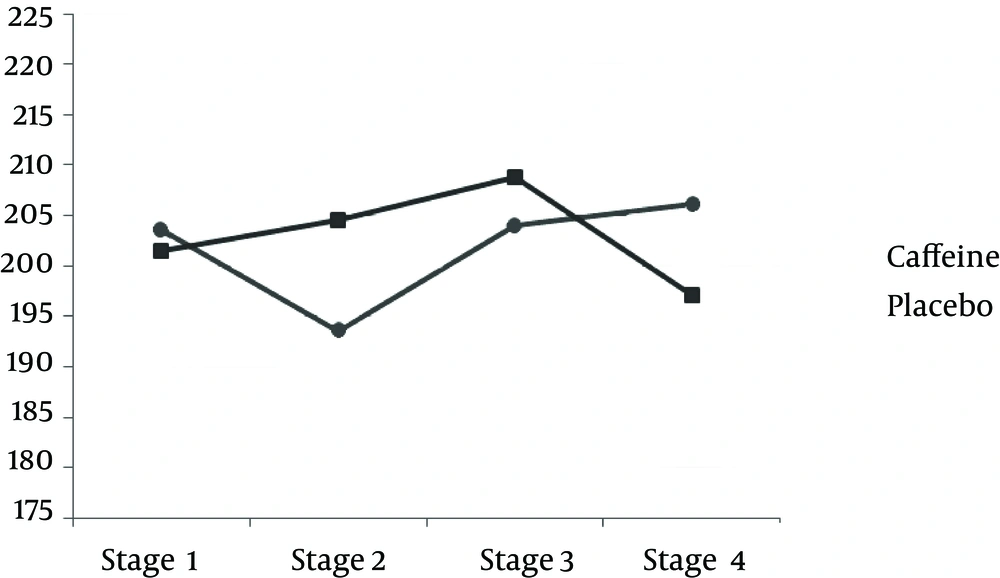
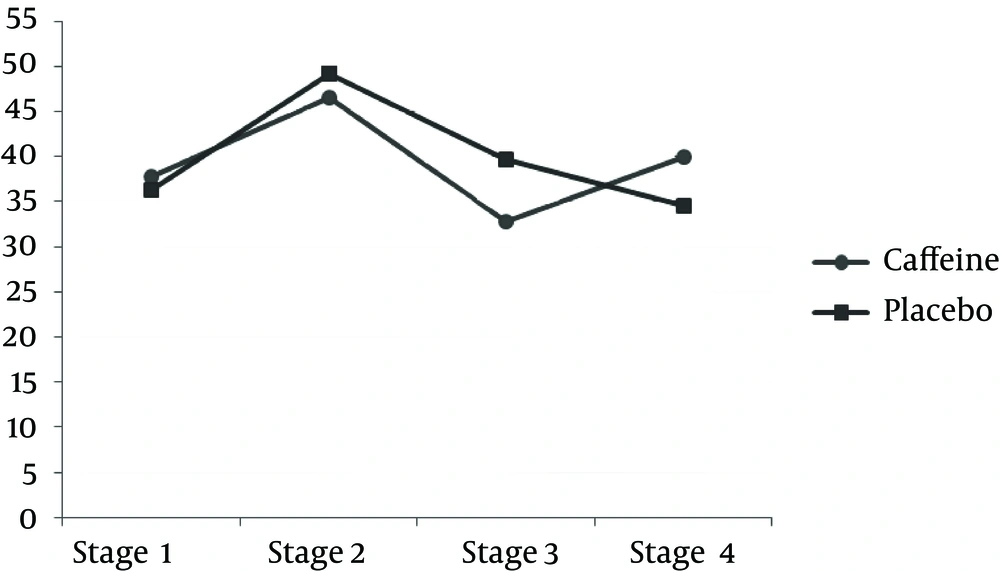
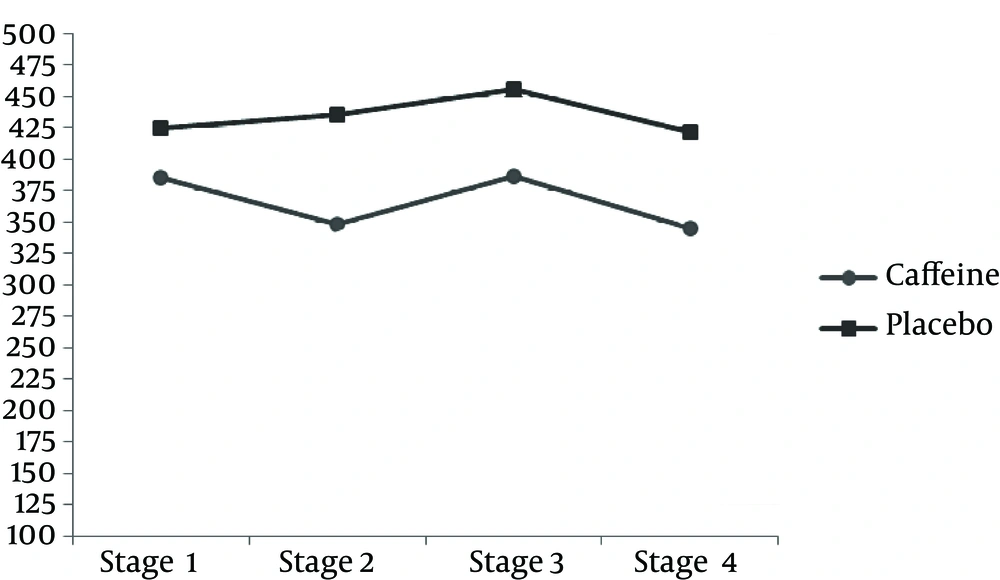
TAC measured after exercise was significantly less in the caffeine group compared to the placebo group. However, after 24 h, the amount of caffeine was significantly more in the caffeine group than the placebo group (P < 0.05) (Table 3 and Figure 1). The results of this study indicate that the MDA concentration after the downhill run was lower in caffeine group compared to the placebo group. But 24 h after running downhill, MDA levels in the caffeine group were significantly increased compared to the placebo group (P < 0.05) (Table 3 and Figure 3). The post hoc Bonferroni test for index of DNA damage also suggests that the values of this index in the third stage were significantly lower than the second stage and also that the fourth stage was significantly lower than the second stage (P < 0.05) (Table 3 and Figure 4).
| Parameters/Inter-Steps | The Difference Between Two Step | P Value |
|---|---|---|
| TTM, nmol/mg protein | ||
| 2 and 1 | 0.015 (0.013) | 1.00 |
| 3 and 2 | 0.073 (0.030) | 0.215 |
| 4 and 3 | 0.098 (0.035) | 0.121 |
| 4 and 2 | 0.025 (0.025) | 1.00 |
| TAC, µM | ||
| 2 and 1 | 0.199 (11.305) | 1.00 |
| 3 and 2 | b 23.697 (6.927) | 0.046 |
| 4 and 3 | b 14.239 (3.658) | 0.022 |
| 4 and 2 | b 37.936 (6.900) | 0.002 |
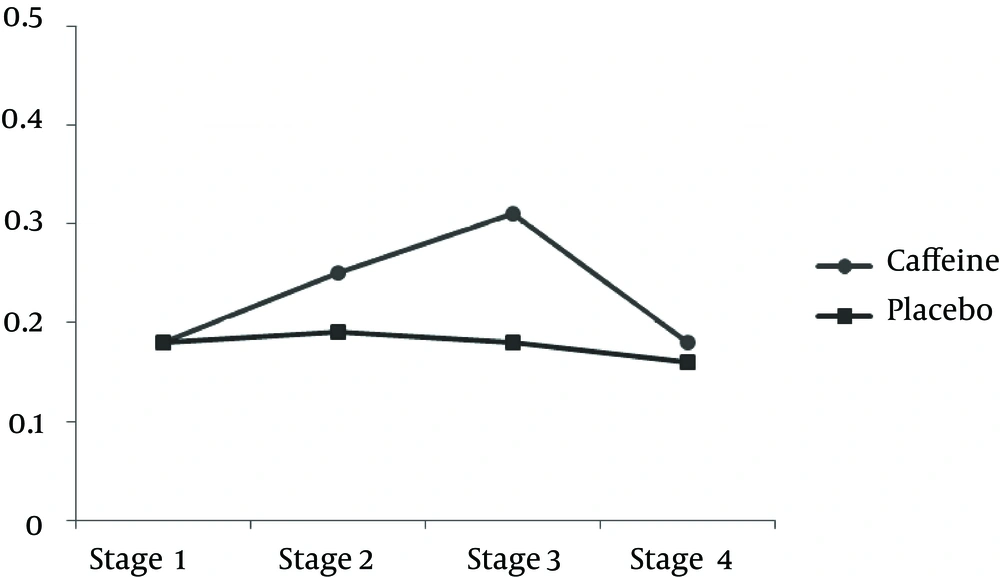
| LPO, µM | ||
| 2 and 1 | 0.845 (0.520) | 0.830 |
| 3 and 2 | b 3.444 (0.403) | < 0.001 |
| 4 and 3 | b 4.823 (1.169) | 0.015 |
| 4 and 2 | 1.379 (0.872) | 0.889 |
| 8oH-dG, pg/mL | ||
| 2 and 1 | 0.604 (1.763) | 1.000 |
| 3 and 2 | 50.026 (13.444) | 0.290 |
| 4 and 3 | 49.628 (8.095) b | 0.001 |
| 4 and 2 | 0.398 (10.590) | 1.000 |
aData are presented as mean (SE).
bSignificant difference.
The results of the Bonferroni post-hoc test indicated that the TAC does not show a significant change in slope in the caffeine group compared to the placebo group immediately and 24 h after exercise (P > 0.05).
5. Discussion
The results of this study indicate that caffeine supplementation has no significant effect on basal antioxidant capacity. But immediately and 24 h after running, the slope of anti-oxidation capacity in both groups did not show significant change. Thus, short-term supplementation of caffeine and the antioxidant capacity of blood running slope changes can affect untrained males.
Many reports have suggested the role of caffeine as an antioxidant in the control of oxidative damage. In vitro studies have reported that mill molar concentration of caffeine typically protects the property against LPO similar to the biological anti-oxide glutathione and even more than ascorbic acid (10-12). Corsetti et al. (2007) reported that rats consuming high amounts of caffeine (16 mg/kg) experienced a reduction in all forms of nitric oxide (NO) production in skeletal muscle and had improved muscle contractile force associated with decreased mitochondrial respiration. Cyclic adenosine Monophosphate, through caffeine concentration, decreased expression of nitric oxide species (5). Varma et al. (2010) and Shi et al. (1991) stated that caffeine (1% of the diet of mice) prevents the oxidation of glutathione (anti-oxidation storage of the eye) in mice via the Fenton reaction and protects the lens from oxidative stress damage (12, 13). Considerable controversy exists in the literature regarding the toxicity of coffee, including its possible carcinogenic and anti-carcinogenic properties.
This study reports on the reaction of 1, 3, 7-trimethylxanthine (caffeine) with the hydroxyl radical (OH), as investigated by electron spin resonance (ESR) trapping. The OH was generated by the Fenton reaction (Fe2+ + H2O2) as well as by the reaction of chromium (V) with H2O2. The results show that caffeine effectively scavenges OH, with a reaction rate constant of approximately 5.9 × 10 (9) M-1 sec-1, which is comparable with those of other efficient OH radical scavengers. ESR measurements provide evidence that a caffeine-derived, oxygen-centred radical is formed in the reaction of caffeine with OH and suggest a biochemical basis for the understanding of the reported anticarcinogenic properties of caffeine and related methylxanthine compounds (12, 13). Glutathione plays an important role in reducing LPO through the elimination of free radicals. Increased levels of oxidative stress cause depletion of glutathione levels. On the other hand, amyloid beta proteins increase cholesterol and oxidative stress. Amyloid beta causes oxidative stress through the production of reactive oxygen species, and it is associated with reduced glutathione depletion. Amyloid beta reducing is associated with reducing oxidative stress and production of reactive oxygen species and reducing glutathione depletion. Caffeine reduces amyloid beta through two mechanisms: the caffeine reduces proteins constituent amyloid beta. The assimilation and degradation of caffeine (Dgradasyon 1) increases amyloid beta. Oxidative stress in the endoplasmic reticulum activates protein transcription factor Gadd153, a protein that sensitizes cells to endoplasmic reticulum stress and produces reactive oxygen species through setting up. The protein Gadd153 is a b-secretase enzyme and will increase amyloid beta. Therefore, caffeine prevents reduction of Gadd153 in the endoplasmic reticulum, amyloid beta production, LPO and, as a result, cell membrane damage (14). Nikolic et al. (2003) have also shown that caffeine reduces LPO levels in the brains of rats (15). All in all, the results of animal studies suggest that a moderate amount of caffeine (10 mg/kg) reduces injury caused by general anaemia and brain anaemia (16).
In agreement with that study, Yadav et al. (2010) reported that caffeine (40 mg/kg) increased LPO levels in young, adult and older mice (16). Olcina et al. (2006) also studied 20 young men, divided into supplement (5 mg/kg caffeine) and control groups, who pedaled to exhaustion on a cycle ergo meter, demonstrating that MDA levels of both groups increased after exercise (17).
Moreover, Olcina et al. (2008) conducted a study in MDA levels that increased after 1 h of cycling exercise at 75% of maximal aerobic power in both placebo and caffeine groups. However, caffeine supplementation did not prevent the increase in serum levels of MDA (18). Exercise training increased muscle citrate synthase activity in the muscle, while in caffeine groups, its activity was decreased in both sedentary and trained rats. Aspartate transaminase levels were increased after training, and caffeine intake suppressed this elevation. Caffeine also diminished alanine transaminase levels in both sedentary and exercised rats. Exercise training induced a significant increase in the activity of the enzymes, superoxide dismutase and glutathione peroxidase, as an increase on thiobarbituric acid-reactive substances levels, while caffeine intake blunted these alterations. Caffeine intake also suppressed liver catalase activity in both sedentary and exercise groups (19). Moreover, an anti-inflammatory effect of exercise was evidenced by reduced plasma MPO activity. In addition, caffeine intake alone and combined with exercise decreased plasma acetylcholinesterase (AChE) and myeloperoxidase (MPO) activities (20). The protective effect of caffeine is due to its antioxidant effect, and consequent maintenance of tissue metabolism was also apparent in its effect against GSH depletion triggered by sodium selenite administration (12). Some of the mentioned studies are in line with the results of our study, while others had contrasting results. It can be mentioned here that severe oxidative stress caused by running downhill and given this dose of caffeine are not able to greatly reduce the effects of oxidative stress-induced activity.