1. Background
Warm-up is generally promoted as an activity that improves athletes’ performance. It is especially important in team sports demanding acceleration, deceleration, or change of direction (1). The general warm-up followed by different kinds of stretching exercises is commonly used. However, some coaches use complex or contrast loading in warm up sessions which involve strength training exercises (isometric or dynamic) followed by biomechanically similar plyometric exercise or vice versa (2). This concept is based on a phenomenon called postactivation potentiation (PAP) that is defined as an acute enhancement of muscular power output produced by performing a preload stimulus before an actual activity (3). It is known that any previous muscle activity can trigger both PAP and fatigue mechanisms (4). Contractile history of a muscle is said to have a positive effect on muscle performance especially in terms of twitch contractions, rate of force development and explosive movements (5).
Recently, there has been an increasing interest in the use of whole body vibration training (WBV) as a warm-up strategy. During WBV, mechanical vibrations produce compensatory muscle contractions as a result of tonic vibration reflex (TVR) via excitation of primary endings of muscle spindles and activation of alpha-motor neurons (6-8). Several acute studies revealed positive effects of WBV on physical performance (9-13). In contrast, other studies showed no beneficial effect of WBV on sprint (14-17), jump (10, 15, 17, 18) and agility (14, 15, 19) performance. The equivocal findings in literature may be explained by methodological differences applied in the studies.
Mechanical loading in WBV is one of the factors that can affect performance. Dabbs et al. (20) suggested that amplitudes between 4 and 10 mm, durations of exposure ranging from 30 seconds to 4 minutes, a work to rest ratio of 1: 1-1:3, rest intervals, ranging from 0 to 10 minutes would be sufficient for optimal performance. Also, when frequency is 50 Hz, amplitude should be around 4-6 mm. In a similar light, Ronnestad (21) proposed that additional external load during WBV may produce larger positive stimulus to the human body and power output especially in well trained subjects. Another factor that can affect dose-response relationship in training schemes is the type of muscle contraction. Researchers suggested that utilization of a single-joint maximum voluntary isometric exercise (22) and a multi-joint isometric exercise (23-25) elicit PAP.
2. Objectives
To the researchers' knowledge, no studies in warm-up settings were administered using WBV with prolonged intermittent low intensity isometric exercises. Such a void in the current literature failed to explain possible mechanisms of isometric contraction in WBV. Thus, the purpose of this study was to compare countermovement jump performance, speed, and agility after loaded and unloaded intermittent low intensity half-squat isometric exercise in vibration and non-vibration conditions in well trained soccer players. The researchers hypothesized that WBV with the extra load would acutely produce greatest gains in jump, sprint and agility in comparison with other preconditioning protocols.
3. Patients and Methods
3.1. Subjects
Twenty-one healthy male college football players (age: 20.14 ± 1.65 years; body height: 179.9 ± 8.34 cm; body mass: 74.4 ± 13.0 kg; % body fat: 9.45 ± 4.8) from the Tuzla University with no history of neuromuscular disease or reported injuries for the past six months volunteered to participate in the study. Athletes trained 8 hours a week (4 sessions of 2 hours each) on field and 3 hours a week (2 sessions of 1.5 hours each) in the gym. Consumption of a light meal at least three hours prior to the beginning of testing sessions was allowed. Hydration taken in small amounts was also encouraged during testing sessions. Avoidance from strenuous activity, tobacco, alcohol consumption, caffeine intake, and sleep deprivation for at least 48 hours prior the testing sessions were also requested. Before the start of the study, subjects were informed about the potential benefits and risks associated with the study. A written informed consent was provided to all subjects. This study was approved by the Ethical Committee of the University of Tuzla, approved with procedures conforming to the principles of the Declaration of Helsinki on human experimentation.
3.2. Procedures
In this study, 5 experimentation sessions, separated by 48 hours, occurred at the Exercise Science Laboratory and in a sport hall of Faculty of Physical Education and Sport, Tuzla University from 8 a.m. to 10 a.m. at the early off-season training period. Day 1 was devoted to measurement of height, weight and percentage of body fat. Body height was measured to the nearest 0.01 m with a portable stadiometer (Astra scale 27310, Gima, Italy). Body weight (BW), body fat percentage was measured by a bioelectric body composition analyzer (Tanita TBF-300 increments 0.1%; Tanita, Tokyo, Japan).
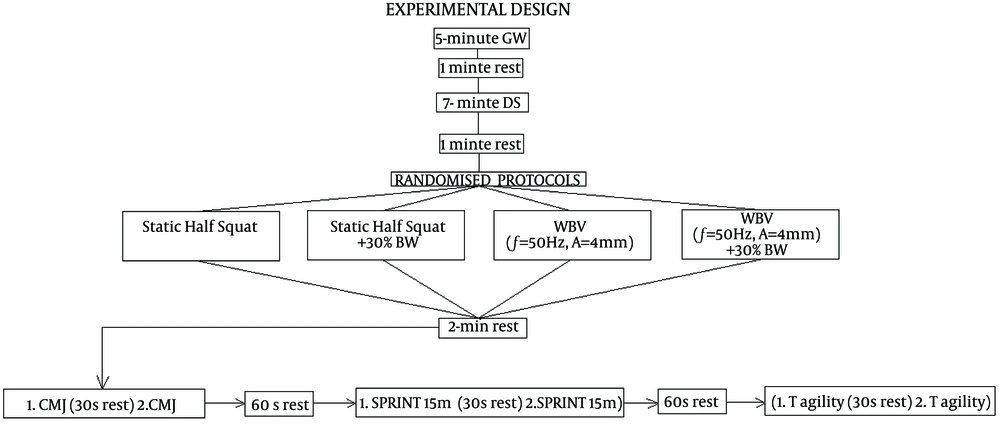
Randomized controlled trials were carried out in the remaining four sessions at which the subjects underwent standing in a static half squat position (ST), ST with 30% of bodyweight (ST + 30%), whole body vibration at f = 50 Hz, A = 4 mm (WBV), and WBV with an additional load of 30% of subject’s bodyweight (WBV + 30% BW). Each intervention was performed 5 times for 60 seconds with a rest interval of 30 seconds in between sets. The subjects stepped off the platform and stood for 30 seconds. The knee flexion angle at the static half squat position was approximately 100 degrees (checked by goniometer before the interventions) with the feet slightly wider then shoulder width apart and the heels on the floor. An Olympic bar (20 kg) and appropriate weighted plates were used to equate a subject's external load of 30% of BW.
All experimental conditions were preceded by a general warm-up (GW) that consisted of five minutes running at a preset pace. This was equivalent to 12 circles around an 86 m circumference area. In the first four circles, the participants had to run 30 seconds per circle. 25 seconds retain was required to finish the second four circles. In the last four circles, the participants had to run 20 seconds per circle. After GW participants had one minute rest and carried on with dynamic active stretching (DS). DS consisted of 7 exercises performed in 7 minutes. Each exercise consisted of 2 sets of 20 seconds with a rest interval of 10 seconds between sets. The rest interval between exercises was 10 seconds. After DS participants had one minute rest before proceeding to one of the experimental protocols.
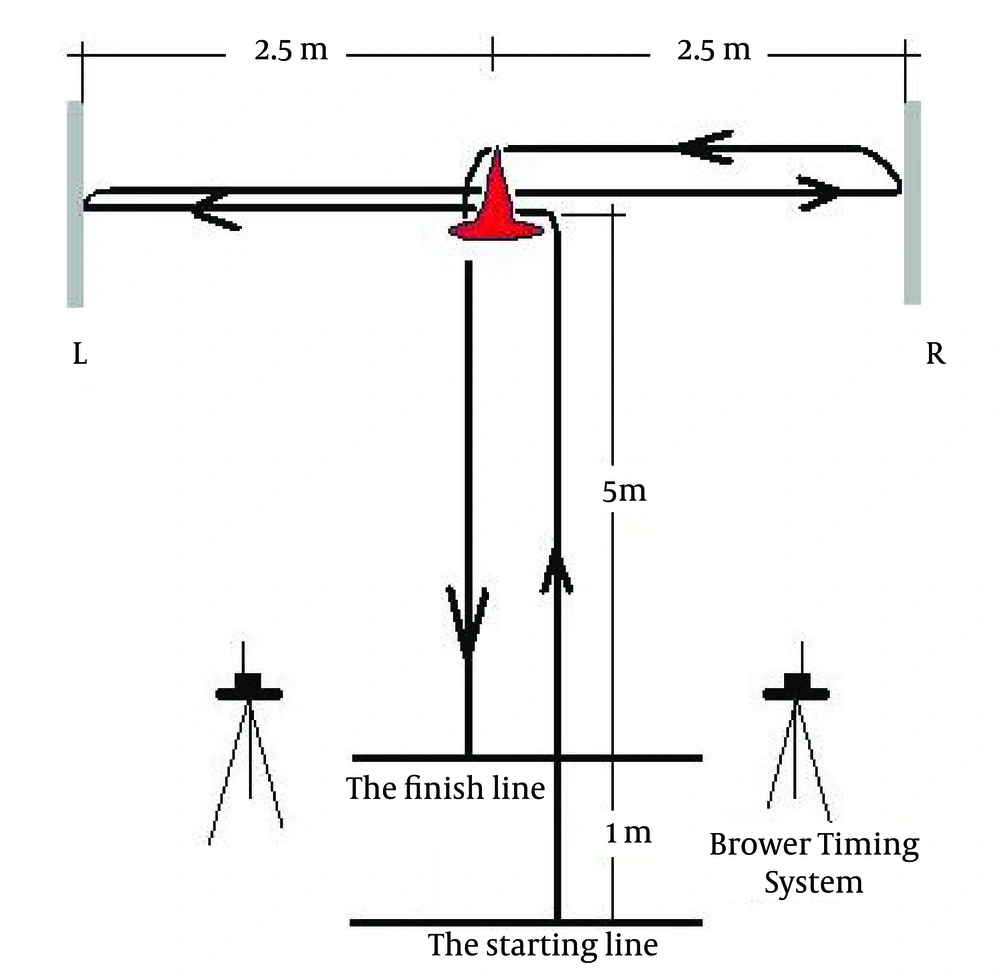
After 2 minutes of an intervention, the participants performed the countermovement jump test (CMJ), 15-m sprint test and the modified agility test. Each test was performed two times. Intratest and intertest rest interval was two minutes. Test-retest reliabilities were 0.96, 0.89, and 0.86 for CMJ, 15-m sprint test and the modified T agility test respectively. In the CMJ, the hands on waist CMJ protocol was used to eliminate the contribution of arm swing in jump performance. For testing the CMJ height, OptoJump System (Microgate, Bolzano, Italy) was used. The participants started in an upright position and executed a countermovement immediately before a jump. The participants were encouraged to land in an upright position, but to bend their knees after landing to reduce mechanical stress. For the 15-m sprint test, the subjects sprinted from a stationary position located 1 m before an automated timing system (Speedtrap II, Brower Timing Systems, Draper, UT, USA). To avoid error, the laser beam was positioned so the height above the ground approximated the height of the subjects’ waist. Once the subjects were prepared, they started on their own decision.
In the modified t-test, the subjects started 1 m behind an automated timing system (Speed trap II, Brower Timing Systems, Draper, UT, USA) and carried out a 5-meter sprint. After the sprint, the participants shuffled to the left (2.5 m) and shuffled back to the right for 5 m. This was continued by a 2.5 m shuffle to the left and back sprint for 5 m. Once the athletes were prepared, they started on their own decision. For the 15-m sprint test and modified t-test, timing sensor was positioned at the height of the subjects’ waist (Figure 1). All players were familiar with the testing procedures because they routinely performed the tests during individual strength and conditioning programs. The subjects were encouraged to make as much effort as possible during all tests. The best trial for each test was recorded for analysis. The experimental protocol is displayed on Figure 2.
3.3. Statistical Procedures
Data are displayed as means and standard deviations. Kolmogrov-Smirnov test showed normal distribution of data. One-way repeated measures ANOVA were utilized to determine significant difference in performance. Mauchly’s test was used to examine the sphericity of data (26). Any violation in sphericity was corrected using the Greenhouse-Geisser estimates of sphericity (27). Eta squared (η2) was used to estimate effect size. Pairwise comparison was determined using Bonferonni post hoc contrast. Analyses were performed using a commercial software (SPSS Inc., Chicago, IL; Version 14.0) with alpha set at 0.05 level of significance.
4. Results
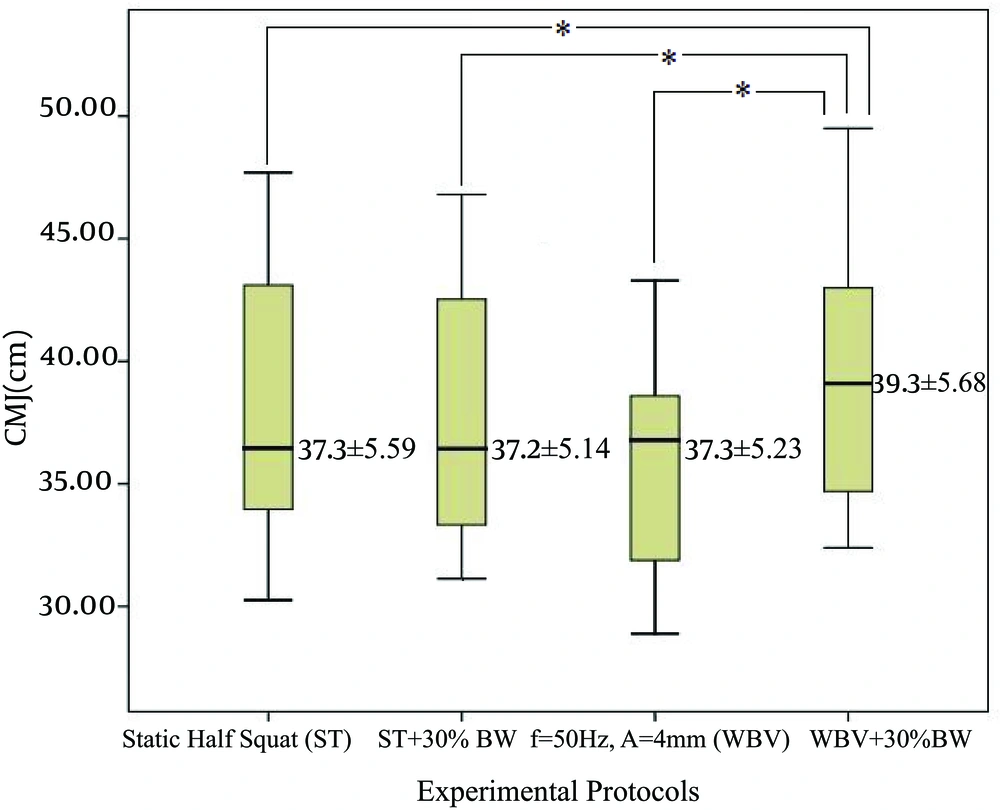
Mauchley’s test for assumption of sphericity on CMJ data was met. Results from the one-way repeated measures ANOVA showed that there was a significant difference in CMJ across interventions, F (3, 60) = 9.06, η2 = 2.21,P = 0.000. Bonferonni post hoc showed that WBV + 30% BW was significantly higher compared to ST (P = 0.008), ST + 30% BW (P = 0.000) and WBV (P = 0.000). Figure 3 shows the effect of various experimental protocols on CMJ performance.
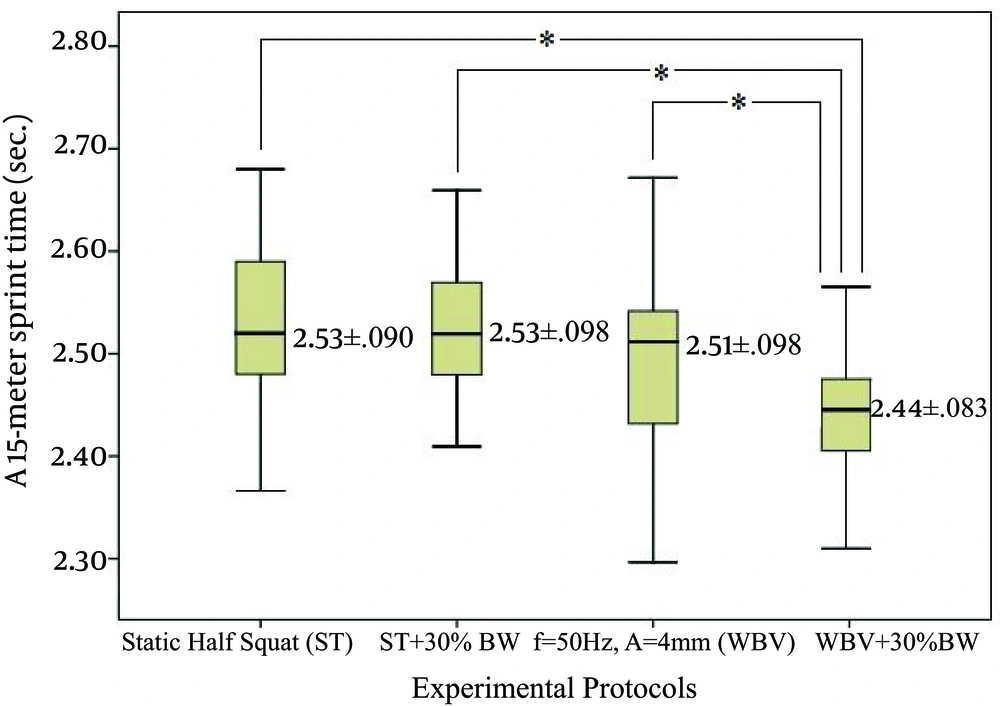
For the 15-m sprint, assumption of sphericity of data agreed with Mauchly’s test. There was a significant difference in sprint time across interventions, F (3, 60) = 23.0, η = 0.865, P = 0.000. Post hoc identified that WBV + 30% BW posted significantly lower time values than ST (P = 0.000), ST + 30% BW (P = 0.000) and WBV (P = 0.000). The effect of experimental protocols on 15-m sprint is displayed on Figure 4.
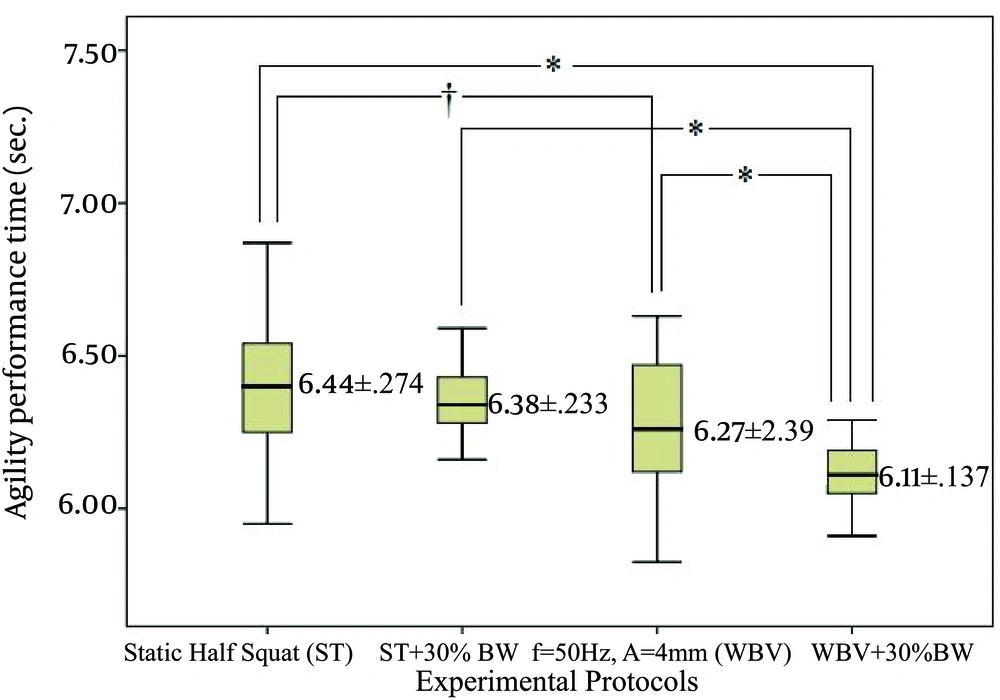
In the modified t-test, Mauchley’s test posted violation in the assumption of sphericity, χ2 (5) = 13.9, P = 0.017. Thus, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity at ε = 0.67 (27). One way repeated ANOVA determined a significant difference in modified t-test time, F (2.01, 40.1) = 21.0, η2 = 0.954, P = 0.000. Post hoc demonstrated that WBV showed lower times than ST (P = 0.013). Also, WBV + 30% BW posted lower times compared to ST (P = 0.000), ST + 30% (P = 0.000) and WBV (P = 0.003). Figure 5 depicts the agility times under various experimental protocols.
Static half squat (ST), static half squat plus 30% of body weight (ST+30%BW), whole body vibration stimulus with f = 50 Hz; A = 4 mm (WBV), whole body vibration stimulus with f = 50 Hz; A = 4 mm and additional load of 30% of body weight (WBV + 30% BW). * Values significantly different from those obtained by the WBV + 30% BW; P < 0.05.
Static half squat (ST), static half squat plus 30% of body weight (ST + 30% BW), whole body vibration stimulus with f = 50 Hz; A = 4 mm (WBV), whole body vibration stimulus with f = 50 Hz; A = 4 mm and additional load of 30% of body weight (WBV + 30% BW). * Values significantly different from those obtained by the WBV + 30% BW; P < 0.05.
Static half squat (ST), static half squat plus 30% of body weight (ST + 30% BW), whole body vibration stimulus with f = 50 Hz; A = 4 mm (WBV), whole body vibration stimulus with f = 50 Hz; A = 4 mm and additional load of 30% of body weight (WBV + 30% BW). * Values significantly different from those obtained by the WBV + 30% BW; P < 0.05. †Values significantly different from those obtained by the WBV; P < 0.05.
5. Discussion
The purpose of this study was to compare leg power, speed and agility exhibited after loaded and unloaded static squat exercise during vibration and non-vibration conditions in male college football players. The main finding of the study showed WBV protocol with additional load of 30% of BW produced the largest gains in CMJ, speed and agility. In addition, WBV treatment showed positive effects on the physical performance compared to control preconditioning protocols without vibration stimulus.
There are no other studies that identified the effects of loaded WBV on jump, sprint and agility performance. It is quite hard to compare the results of this study to some recent studies because of methodological parameter differences. In the current study, intermittent (5 × 60 seconds) WBV protocol with a 30-second rest ratio was used with high frequency (50 Hz) and medium amplitude (4 mm). Most of the similar available studies utilized continued WBV protocols (30-90 seconds) in combination with low/high frequencies/amplitudes (28-30) or intermittent protocols, but with smaller number of applied bouts of the vibration stimulus (10, 13-15).
In jumping, WBV + 30% BW showed greatest gains on CMJ than WBV, ST + 30% BW and ST protocols. No significant differences between WBV, ST + 30% BW and ST protocols were observed. This is somewhat in line with the findings of Adams et al. (2009) which demonstrated those high frequencies (40 Hz and 50 Hz) were most effective on CMJ performance when applied in combination with high amplitudes (4-6 mm) and evaluated between first and fifth minute post treatment (28). Also, Armstrong et al. (30) showed significant improvement in countermovement jump height after WBV at 5 minutes and 10 minutes post treatment from varying frequencies (30, 35, 40 or 50 Hz) and amplitudes (2-4 mm or 4-6 mm) in male and female college students. In contrast, the other studies (10, 15, 17, 18) showed detrimental effects or no change in vertical jump after vibration stimulus and as it was mentioned before these equivocal findings may be a result of different WBV protocols used.
The comparative results of different preconditioning protocols on sprint performance were similar to those obtained in jumping performance. WBV + 30% BW protocol showed superior effects on sprint performance compared to WBV, S + 30% BW and ST. No significant differences between WBV, ST + 30% BW and ST protocols were observed. Although not statistically significant, WBV protocol showed better effects on sprint performance compared to non-WBV protocols. This is to some extent in agreement with the studies of Ronnestad et al. (31) who reported enhancement in 40-meter sprint performance in male football players after a 30-second WBV protocol (f = 50 Hz; A = 3 mm) compared to a control. Furthermore, WBV at a frequency of 30 Hz did not show any improvement in the sprint performance compared to control. In follow-up study, Ronnestad et al. (32) demonstrated an on-ice sprint performance enhancement one minute after WBV preconditioning (f = 50 Hz; A = 3 mm) in ice-hockey players. Contrastingly, several studies failed to provide evidence that acute WBV stimulus positively affect sprint performance. Gerakaki et al. (17) reported that WBV (90 seconds, 50 Ηz, 2 mm) did not lower a 60-meter sprint time as well as it did not affect a step length and rate. Cochrane (2013) (14) determined that the intermittent (5 × 1 minute) WBV (26 Hz, 6 mm) treatment with one minute rest time between; affected 1.5 m sprint time compared to control group, but there was not noticeable effects between pre and post conditioning sprint times. Similarly, Kavanaugh et al. (16) and Roberts et al. (33) used a single WBV bout of 30 and 60 seconds, with vibration (50 Hz and 3 mm) and 26 HZ and 4 mm respectively and found no significant improvement in 30 m sprint performance between WBV and sham. Bullock et al. (13), who used intermittent (3 × 60 seconds with 180 seconds of relief period between) WBV protocol with vibration stimulus of 30 Hz and 4 mm, reported no effects on 30 m sprint performance in international skeleton athletes. One year later, Bullock et al. (34) tried to elicit acute potentiation with higher frequency (45 Hz) and shorter rest period (60 seconds) between vibration stimuli, but they obtained the results similar to the previous study.
Preconditioning protocols on agility performance demonstrated significant differences between the WBV + 30% BW and other protocols. Additionally, WBV showed significant difference on agility compared to ST. Despite the widespread use and popularity of WBV and importance of agility in team sports, there is limited number of studies that investigated the acute effects of WBV on agility performance. Cochrane (14) investigated the effect of the intermittent (5 × 1 minute; with 1 minute rest) WBV protocol (26 Hz, 6 mm) on reactive agility. The results showed no significant changes for the reactive agility test. Concomitant findings were presented by Pienaar (35) which included WBV protocol before dynamic hockey-specific warm-up trying to investigate its effects on T-agility test (ATT) performance. The study used two sets of five WBV exercises with the first set using a low amplitude (2-4 mm) for 30 seconds. The second was increased to a higher amplitude (4-6 mm) and duration of 45 seconds. Both sets of WBV stimulus were set at frequency of 35 Hz. Athletes were allowed 30 seconds of relief period between exercises and two-minute rest in between sets. Although the results did not show any significant acute effect of WBV on agility performance, improvements in applied ATT time were recorded. In a similar vein, Torvinen et al. (19) also reported no significant effects of four minutes of WBV stimulus with a progressive (every 1 minute) increment in vibration frequency (from 15 Hz to 30 Hz) on shuttle run (change of direction and agility test) measured 2 and 60 minutes after treatments. Conflicting results with the findings of the study could be explained by various agility measurement protocols. The new modified T agility test used in this study covers shorter distances which was different in the previous researches. It is known that leg power has strong correlation with short distance agility performance (36). This may suggest that improvement in power performance (CMJ) also affected the results in the modified T agility by generating leg power in a short period of time.
Performance gains in CMJ, sprint and agility after WBV with additional load can be attributed to an increase in muscle temperature (37) and blood flow (38). It is well documented that the higher muscle temperature increases nerve conduction velocity, elevates muscle enzyme activity, increases dilatation of blood vessels and blood flow to the activated muscles that provides better muscle oxygenation during work (39-43). In a study by Rittweger et al. (44), they found out that application of 40% of external load with bodyweight under WBV increased specific oxygen uptake when compared to bodyweight WBV alone. Specific oxygen uptake was enhanced by increased muscle kinetics. In addition, another possible explanation for the superiority of the loaded WBV (50 Hz and 4 mm) maybe related to increased tonic vibration reflex (TVR) conditions that led to better muscle capabilities; dampening external perturbations and reducing resonance effects. During WBV the mechanical vibrations produce compensatory muscle contractions as a result of TVR via excitation of primary endings of muscle spindles and activation of alpha-motor neurons (6-8). Another possible mechanism that contributed to the results may be linked to the larger presence of post activation potentiation (PAP) at WBV + 30% BW compared to other interventions. PAP refers to improvement in muscle performance preceded by muscle activity which can be assessed by twitch potentiation or reflex potentiation (2). However, researchers suggested that PAP that may result by WBV may be related to twitch potentiation rather than reflex potentiation (11, 45). WBV was reported to increase muscle activity and the neuromuscular stimulus compared with no-vibration conditions (6, 21). Exercise intensity is reported to be an important factor in PAP. Lowery et al. (46) suggested that high-intensity workloads may prolong the duration of PAP, while Behm et al. (47) and Vandervoort et al. (48) reported higher magnitude of PAP with increased exercise intensity. Luo et al. (49) found out that 10% and 30% load 1RM increased rectus femoris, vastuslateralis, and vastusmedialis electromyographic parameters under WBV. This may suggest that the additional load could lead to the higher PAP magnitude, as well as to more increased motor unit recruitment and synchronization (50) compared to WBV without extra load and low intensity intermittent isometric protocols.
Lastly, the findings of the study support the proposition of Ronnestad (21) that additional external load in WBV may facilitate larger stimulus than the unloaded WBV especially in well trained athletes. The subjects in the current study were well trained football players with well-developed muscular endurance and fatigue resistance. Additional load during WBV showed beneficial for their physical performance, but it could not be purported if the protocol would be valuable for other types of athletes and to what extent. Current limitations of the study include quantification of muscle activity and body temperature measurement across interventions to facilitate better understanding of the various dose-response mechanisms in the study. The study also failed to compare WBV interventions with traditional warm-up strategies.
The findings of the study supported the hypothesis. It can be concluded that WBV stimulus with additional extra load of 30% of body weight improved jump, sprint and agility performance compared to other unloaded preconditioning protocols. In practice, this information is very important because the WBV with the additional load can be added to the regular warm up session of football players to enhance muscle activation, increase body temperature and blood flow that is to improve physical performance in acute settings. Also, this kind of WBV stimulus may be used in combination with resistance strength training in order to produce long-term effects and adaptations in the neuromuscular system. Lastly, low intensity intermittent isometric protocols can be used as an alternative warm-up strategy in situations where WBV training is unavailable.