1. Background
Cancer is a major cause of morbidity and mortality throughout the world (1). Cancer treatment is directly related to the type and stage of the disease, age and medical history of the patient. Chemotherapy is one of the most common treatments (2, 3) and cisplatin (CP) as a member of the platinum drugs is used to treat testicular, ovarian, breast, lung, and bladder cancers (4). CP has numerous side effects such as liver and testis injury (5). The major side effect of CP is nephrotoxicity (6). CP is the inhibitor of desoxyribonucleic acid synthesis (4) and nephrotoxicity of CP seems to be related to reactive oxygen species. Reactive oxygen species, especially the hydroxyl radical, induce lipid peroxidation and cell membrane damage and reduce glomerular filtration rate (GFR) (7-9). It is reported that CP-induced nephrotoxicity may be ameliorated by scavenging agents such as vitamins E and C, losartan, melatonin, selenium, superoxide dismutase, and erythropoietin (10-16). Researches indicate that exercise activity changes antioxidant content (17-19), and as an example, it reduces antioxidant levels of erythrocyte GSH-PX (17). Also, moderate regular exercise decreased malondialdehyde (MDA) levels (20). Another study showed long-term aerobic exercise increases glutathione erythrocyte peroxidase enzyme (GSH) activity and superoxide dismutase (SOD) activity (18). Physical exercise has been suggested in cancer patients undergoing chemotherapy (21, 22) to improve mental and physical activities and rehabilitation, because exercise increases heart-respiratory fitness and muscular strength, and improves body composition. Moreover, exercise reduces fatigue, anxiety, and depression in these patients (23). Accordingly and considering the beneficial effects of exercise, it seems that exercise can affect CP-induced nephrotoxicity.
2. Objectives
The current study was designed to investigate the protective effect of aerobic exercise against CP-induced nephrotoxicity.
3. Materials and Methods
3.1. Experimental Protocol
Thirty adult male (weighing 211 ± 7 gr) Wistar rats (Animal Centre, Isfahan University of Medical Sciences, Isfahan, Iran) were used in this research. The animals were given free access to water and rat chow and housed at a temperature of 23 – 25°C under a 12 hours light/ 12 hours dark schedule. The experimental procedures were approved in advance by the Isfahan University of Medical Sciences Ethics Committee.
The rats were randomly assigned to four groups. The animals in group I (called EX + CP + EX) had aerobic exercise on a treadmill one hour per day and five days per week for eight weeks. Then, the exercise protocol was continued for another week, but during this week, the animals also received CP (2.5 mg/kg/day; ip). Group II (called EX + CP) had protocol the same as group I without exercise in the last week during CP therapy. Groups III and IV were assigned as positive (CP) and negative control (called sham) groups, and they were treated with CP and saline, respectively, without exercise. The animals in the control groups were placed in the apparatus for the same duration of time without running.
3.2. Treadmill Exercise
The rats were subjected to treadmill exercise as a moderate regular exercise on a motorized rodent treadmill (Isfahan University of Medical Sciences). To this end, the animals were placed on the apparatus and forced to run at the speed of 16 m/min for 15 minutes for 7 days. The animals that could not run were excluded from the study. After one week of adaptation, groups I and II received a progressive exercise. During the first two weeks, the speed was increased to 20 m/min for 60 min/day for 5 days/week, the third and the fourth weeks to 23 m/min, the fifth and the sixth weeks to 25 m/min, and the last two weeks to 28 m/min. After endurance training for eight weeks, group I with reduced aerobic exercise to 23 m/min and group II with stopped aerobic exercise were treated with CP for one week. The angle of inclination was 0% during the study. This method was moderate exercise and it is mentioned that oxygen consumption in this position was about 65% for the rats (24, 25).
3.3. Measurement and Histopathological Procedures
All animals were weighed during treatment with CP. At the end of the experiment, the rats were anesthetized with chloral hydrate injection (450 mg/kg; ip) and blood samples were taken from the heart. Then, the animals were sacrificed and right and left kidneys were removed and weighed. The blood samples were centrifuged at 6000 rpm. Then, serum was collected and stored at -20ºC until measurement. The left kidney was fixed in 10% neutral formalin solution and embedded in paraffin for histopathological staining. The assessment was conducted by a pathologist who was blinded to study protocol. The changes in kidney tissues were recorded using a grading scale of 0 - 4 which was based on a subjective impression of the changes (tubular dilatation and simplification, tubular cell swelling and necrosis, tubular casts, and intraluminal cell debris with infiltration of inflammatory cells). The kidney tissue damage score (KTDS) was assigned as zero score for normal tissue, score of 1 for ≤ 25% damage, score of 2 for > 25% and ≤ 50% damage, score of 3 for > 50% and ≤ 75% damage, and score of 4 for > 75% tissue damage. The right kidney was homogenized and centrifuged. Then, the supernatant was stored at -20ºC for measurement. The levels of serum creatinine (Cr) and blood urea nitrogen (BUN) were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum and kidney levels of nitrite (stable NO metabolite) was measured using a colorimetric assay (ELISA) that involves the Griess reaction. The serum and renal levels of MDA were measured manually (26). Bodyweight (BW) of the animals was also measured during the last week of CP therapy.
3.4. Statistical Analyses
Data were expressed as mean ± SEM. One-way ANOVA (followed by the LSD as post test) was applied to compare the BUN, Cr, NO, and MDA levels; and kidney weight (KW) and bodyweight (BW) between the groups. To compare the KTDS between the groups, Kruskal Wallis and Mann-Whitney tests were applied. P < 0.05 was considered statistically significant.
4. Results
4.1. Effect of CP and Exercise on Serum Levels of BUN and Cr
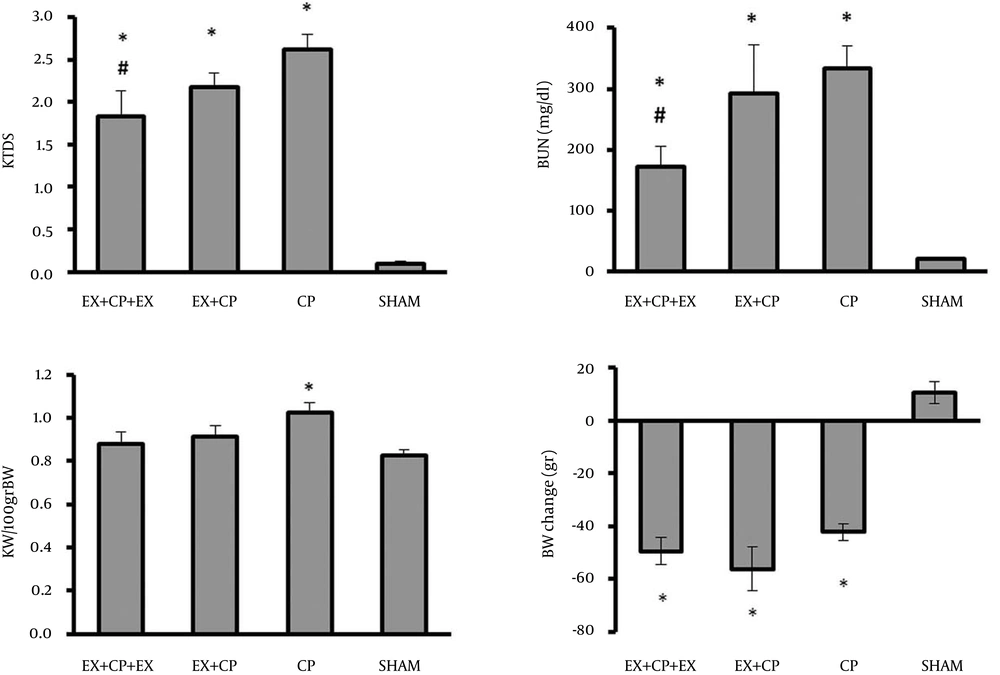
The serum levels of BUN and Cr significantly increased in the positive control group (CP group) when compared with the negative control group (sham) (P < 0.05). These parameters were significantly reduced in EX + CP + EX group when compared with the positive control group (P < 0.05), but by stopping exercise in group II during CP therapy, no beneficial effect was seen on serum levels of BUN and Cr (Figure 1).
4.2. Effect of CP and Exercise on BW Changes, KW, and KTDS
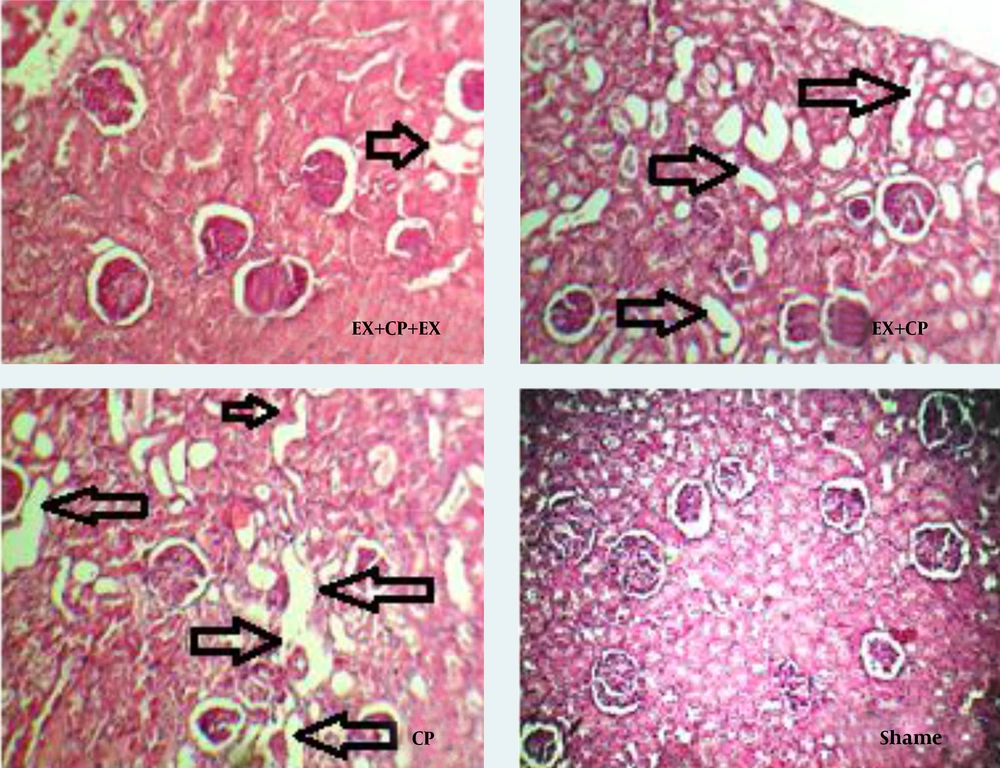
CP alone reduced BW in the positive control group when compared with the negative control group (sham) (P < 0.05). Exercise had no significant effect on BW. The KW significantly increased in the positive control group when compared with the sham group (P < 0.05). No significant changes in KW were observed in groups I and II in comparison with the negative and positive control groups. KTDS was evaluated and scored by a pathologist. Higher kidney damage score was detected in the positive control group when compared with the negative control group (P < 0.05). The KTDS in EX + CP + EX and EX + CP groups significantly reduced when compared with the positive control group (P < 0.05) (Figure 1). The images of kidney tissues is shown in Figure 2.
4.3. Effect of CP and Exercise on Nitrite and MDA Levels
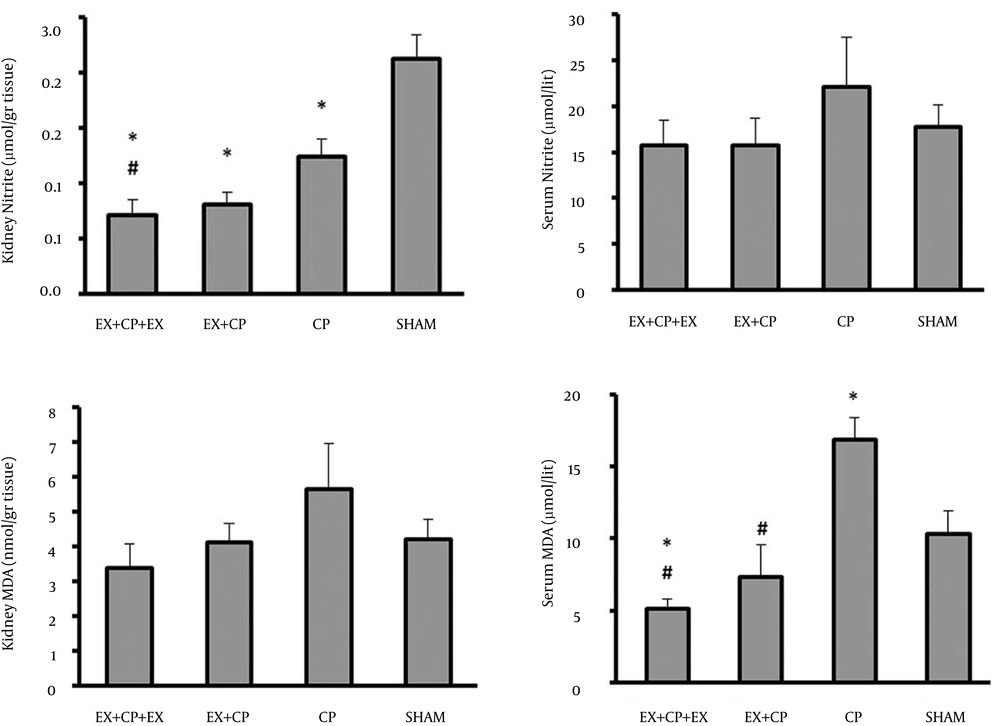
The serum level of nitrite did not alter significantly between the groups. This is while the kidney level of nitrite reduced significantly (P < 0.05) in all CP-treated groups when compared with the sham group. However, this reduction was more in EX + CP + EX group, and accordingly the kidney level of nitrite in group I was significantly reduced by exercise when compared with the positive control group (P < 0.05) (Figure 3).
The serum levels of MDA significantly increased in the positive control group (CP) when compared with the negative control group (sham) (P < 0.05). Exercise could ameliorate the serum level of MDA in both EX + CP + EX and EX + CP groups (P < 0.05). However, no significant change in kidney MDA level was observed between all the experimental groups (Figure 3).
5. Discussion
The main objective of this study was to investigate the role of aerobic exercise in reducing CP-induced nephrotoxicity. In our study, CP increased serum levels of Cr, BUN, which was confirmed by pathological data. Our findings were in agreement with previous studies (14, 27-31). Histopathological examination showed that CP induces massive degenerative changes in 50 - 75% of glomeruli and renal tubules (32) by mechanisms such as oxidative stress and apoptosis, and our histopathological investigation indicated that moderate regular exercise reduced KTDS. Accordingly, BUN and Cr were also decreased by exercise. It has been reported before that exercise can improve kidney function by improving metabolic factors and glomerular filtration rate and reduction of albuminuria (33-35). It seems that exercise during CP therapy could reduce the CP-induced oxidative stress and prevent renal dysfunction. Moreover, exercise potentially could improve endothelial function and CP is known as a drug that causes endothelial injury and impaired endothelial function by decreasing eNOS (36). However, our data did not support this idea when the serum level of nitrite was analyzed. The CP alone therapy not only decreased the serum nitrite level but also increased it insignificantly. Possibly, the increased NO metabolite (nitrite) by CP was not related to eNOS, and it may be produced from other NO formation pathways such as iNOS. Different data for nitrite level in the kidney tissue have been found. We observed that CP decreased the kidney level of nitrite and exercise decreased it more. Therefore, we can conclude that exercise in a CP-treated animal potentially could decrease both serum and kidney levels of nitrite when compared with the non-exercise group, but the importance should be defined in future studies. In our study, CP injection increased the serum MDA level and moderate regular exercise improved this factor. It is reported that appropriate exercise increases antioxidant enzymes (37) and decreases MDA level (20, 38). Furthermore, treatment with antioxidants reduces CP-induced nephrotoxicity (13, 14, 27, 30, 36, 39). It seems that the positive effect of aerobic exercise on antioxidant defense system can reduce CP-induced nephrotoxicity. In spite of the beneficial effect of moderate regular exercise on kidney function, it had no beneficial effect on body weight. A study showed that physical activity decreased body weight (40-42), and it is reported that administration of CP increased kidney weight, which is directly related to kidney injury (43).Our study indicated physical exercise either before CP therapy or simultaneously with CP therapy may have positive effects on reducing the nephrotoxicity induced by CP. Aerobic exercise may reduce CP-induced nephrotoxicity with a favorable effect on renal function or increasing activation of antioxidant system.