1. Background
The two main arms of the stress system include the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis. These two systems coordinate the response of many physiological systems, bringing the body to homeostasis. There is solid evidence from studies in both animals and humans that stress results in the concomitant activation of cells from the nervous, and endocrine systems and in the release of diverse biologically active compounds, including catecholamines and glucocorticoids (1-3). Dysregulation of either of these stress systems can lead to a maladaptive response to stress (4).
Activation of the SNS due to stress is measurable by various parameters such as galvanic skin responses (GRS), secretion of salivary alpha-amylase (sAA), and heart rate (HR). GSR is a change in electrical conductivity between two points on the respondent’s skin. Fahrenberg and Wientjes (5) report GSR to be the most convenient physiological indicator for workload and GSR amplitudes may reflect the amount of affective or emotional arousal elicited by a stimulus or a situation.
The results of studies on animals and humans indicate that the SNS plays a powerful role in the secretion of sAA, with contributions of both alpha-adrenergic and beta-adrenergic receptors. Thus, sAA can be considered as an indicator of the sympathetic activity (6).
The HPA axis is a neuroendocrine system involved in maintaining homeostasis in mammalian organisms under physiological conditions and stress, and cortisol is the principal hormone of HPA axis in humans (7, 8). Salivary cortisol is an easily obtainable biofluid and noninvasive source for evaluating the HPA axis.
Resilience refers to the ability to cope with stressful events and the parachute jumping can be a stress model to study emotional and physical stress in humans (9, 10). There are studies on aggressive behavior that indicate a diverse stress response between SNS and HPA (10). Since sportive activity simulates some situations of aggressive behavior, the purpose of this study was to investigate whether the SNS and the HPA axis would show parallel or divergent stress response patterns in a session of first parachute jump. In general, there are no studies about the effect of skydiving on both the SNS and HPA axis.
2. Objectives
Stress induces an activation of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis. The purpose of this study was to investigate whether the SNS and the HPA axis would show parallel or divergent stress response patterns in a session of first parachute jump.
3. Patients and Methods
3.1. Subjects
Seven healthy adult male sport-parachutists, with the same level of experience, took part in the study. All participants were in good health, as defined by the absence of cardiovascular disease, and no history of endocrine disorders and were not taking any medication. Prior to data collection, the purpose of this study was explained thoroughly and informed consent was obtained from each participant, according to the Declaration of Helsinki. The study has been approved by the Ethics Committee of the Second University of Naples. The sample size is conditioned by the fact that it is preferable to test the reactions in the same day with identical environmental conditions and weather.
3.2. Study Protocol
The study protocol consisted of one day of testing in which each participant was asked to continue his normal work and leisure activities. The subjects were instructed to have food and beverages as usual and to sleep a sufficient amount of hours. Jumps were performed between 12:45 and 13:15. The altitude reached by the aircraft at the time of the jump was 450 meters, allowing for a freefall of 5 seconds before the parachute was opened and approximately 100 - 110 seconds until touching the ground.
3.3. Hormonal Assay
Salivary samples were collected between 9 and 11 twice at an interval of 120 minutes (basal), within 60 second (1 minute post-jump); and 90 minutes after touching the ground (90 minutes post-jump), by means of cotton-swabs (Salivette, Sarstedt, Rommelsdorf, Germany). Participants were asked to place the cotton swab into their mouth for at least 2 minutes while chewing and then insert it back into a special plastic tube. Samples were returned as soon as possible to the laboratory and stored at -20°C for further free cortisol and sAA assay. The absence of blood contamination was checked by a salivary blood contamination kit (Salimetrics LLC, State College, PA, USA). The saliva collecting tubes were centrifuged at 1500 g for 15 minutes at 4°C. Fifty to 100 µL of saliva was used for duplicate analysis. All samples were tested in the same series to avoid any variations between tests. The cortisol and sAA concentrations were measured by commercial kits (Salimetrics LLC, State College, PA, USA), according to the manufacturers’ instructions. The intra-assay and inter-assay coefficients of variation were 3.7% and 7.5% for cortisol, as well as 4.2% and 7.8% for sAA. A standard plate reader (Power Wave XS, Bio-Tech Instruments, US) was used for salivary determination at 450 nm.
3.4. Measurement of HR Responses and GSR
Within less than 5 minutes following saliva collections, HR responses and GSR data were recorded. Each participant wore a chest belt hard-wired to a digital R-R recorder (Polar Electro Oy, Kempele, Finland), where the QRS-signal wave-form R-R signal was sampled at the resolution of 1 ms. The HR (beats minutes -1) was calculated according to the following formula: HR = 60 x R-R interval-1, where R-R interval was converted into seconds. The GSR parameters were measured simultaneously using the SenseWear Pro Armband™ (version 3.0, BodyMedia, Inc. PA, USA), which was worn on the right arm over the triceps muscle at the midpoint between the acromion and olecranon processes, as recommended by the manufacturer.
3.5. Statistical Analysis
The statistical program Graph Pad Prism for Windows (v.5.01) (San Diego, CA, USA) was used for the analysis and treatment of the data. Physiological responses during the experimental session were evaluated using one-way analysis of variance (ANOVA) with repeated measurements, as well as the Student’s t-test, when appropriate. When a significant F value emerged (P < 0.05), the Bonferroni multiple comparisons test was performed to compare each group with the other ones. Data are reported as Mean (M) ± Standard Deviation (SD).
4. Results
4.1. Physical Characteristics
The physical characteristics of the subjects participating in the study are presented in Table 1.
4.2. Physiological Responses to Stress
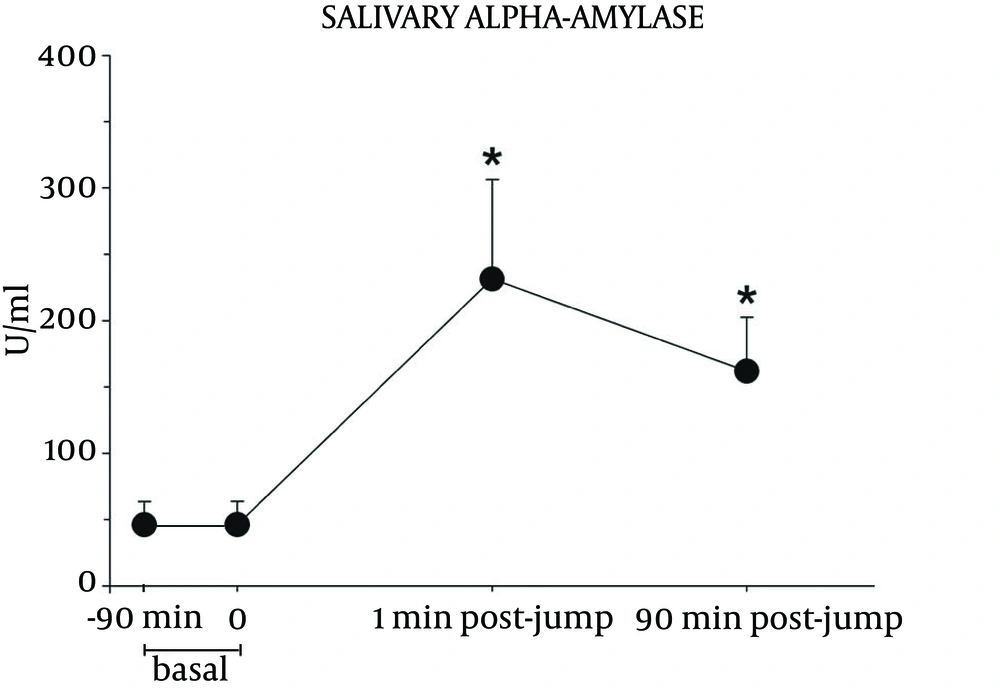
Parachute jumping induced significant increases in both autonomic and hormonal variables. The analysis of variance showed significant differences between the experimental conditions for sAA (F (2, 11) = 4.7, P < 0.05], GSR [F (2, 11) = 4.2, P < 0.05], HR [F (2, 11) = 21.7, P < 0.01], and cortisol [F (2, 11) = 7.9, P < 0.01)]. Figure 1 reports the change in sAA. This variable reached a peak immediately after landing and it remained significantly elevated at 90 minutes after touching the ground, compared to basal values.
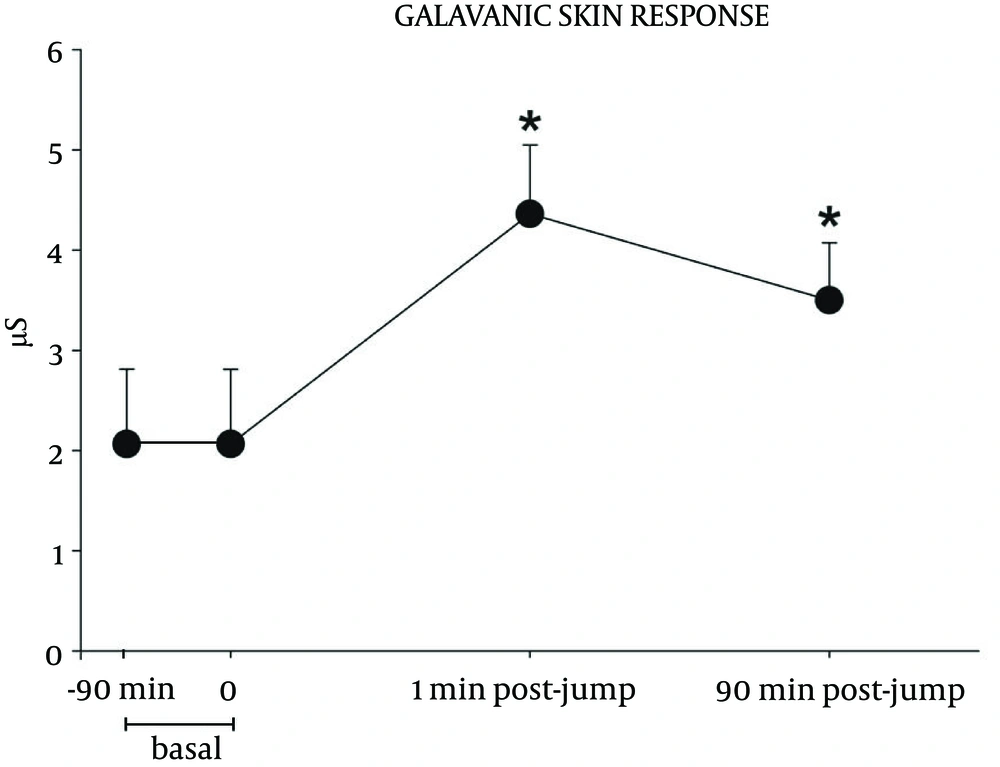
Figure 2 shows the variations of GSR. This variable reached a peak immediately after landing and it remained significantly elevated at 90 minutes after touching the ground, compared to basal value.
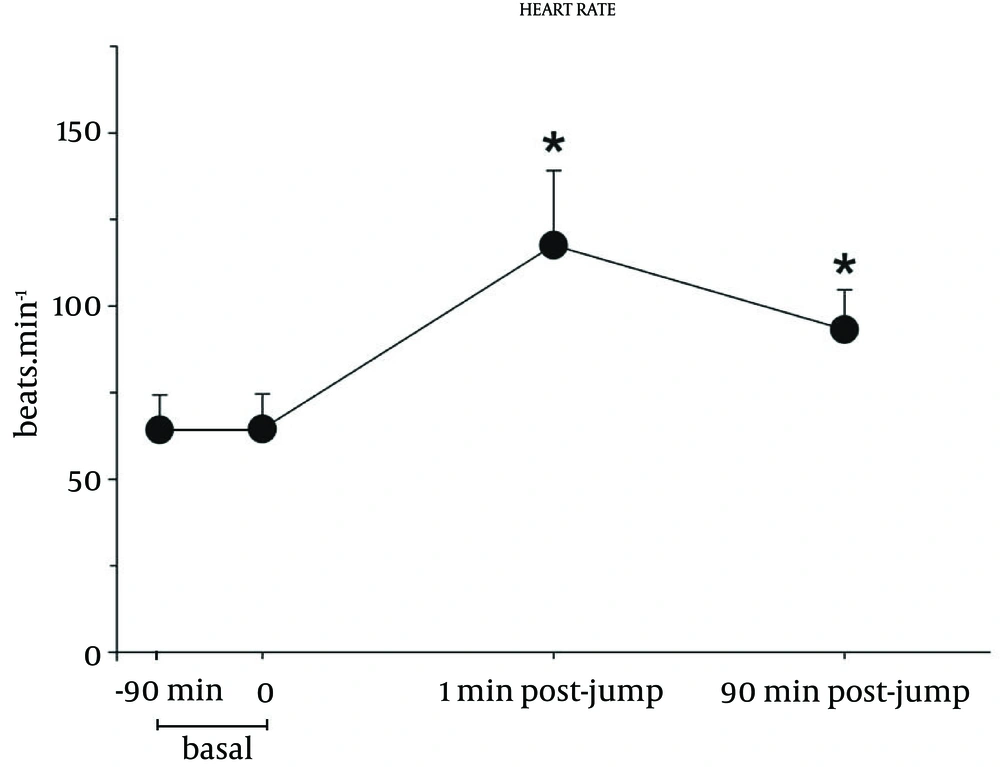
Figure 3 shows the changes in HR, which significantly increased from a basal mean of 67.4 ± 9.8 beats minute-1 to a peak value of 120.3 ± 20.7 beats minute-1 at the jump, corresponding to 51.4 ± 13.2% of the individual’s maximal HR. It remained significantly elevated at 90 minutes after touching the ground, compared to basal value. No ventricular arrhythmias or ST segment abnormalities were recorded.
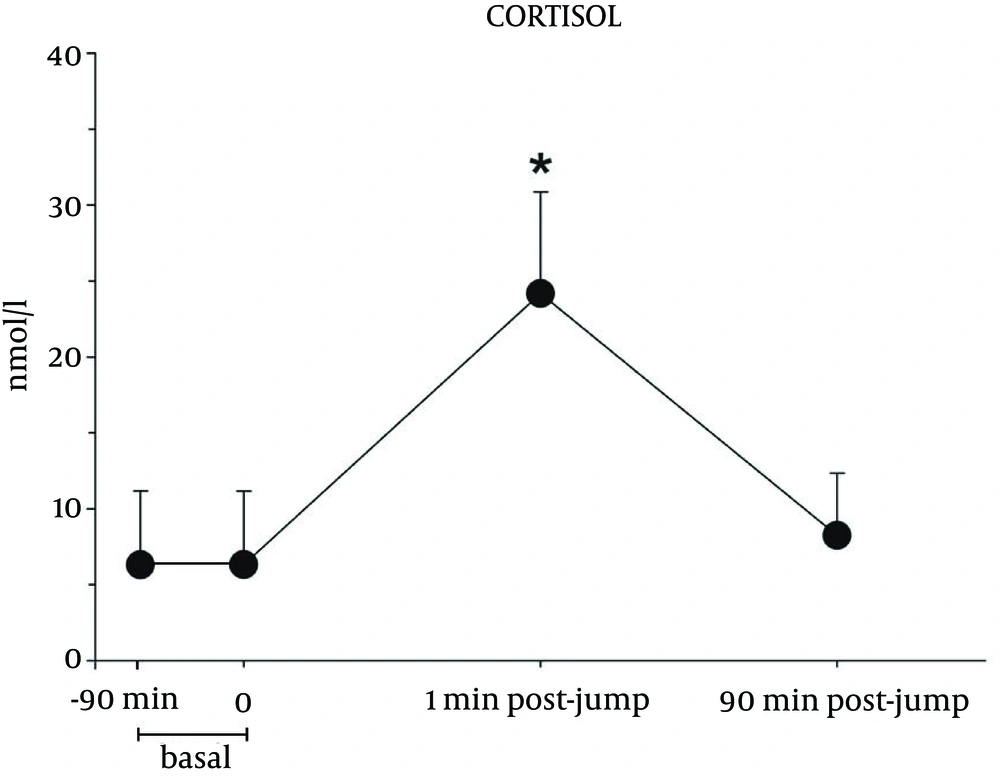
Figure 4 reports the concentration of cortisol, which increased immediately after landing. Cortisol returned to basal values within 90 minutes. The same values on basal times (-90 minutes and 0) suggest that the monitored variables do not change in a short period. This justifies the lack of the control group.
5. Discussion
To our knowledge, this study is the first experiment that investigated both the SNS and HPA axis in first-time parachutists. These evidences suggest that parachute jumping is accompanied by a distinct pattern of response in SNS and HPA activities.
Given the connections between the two systems, symmetry between their activities makes sense. In fact, the lateral paraventricular nucleus (PVN) connects to areas in the hindbrain responsible for the sympathetic activity via neurons that secrete CRH (11-13). Moreover, catecholaminergic pathways project from the locus coeruleus (LC) to the PVN (14). Experimental manipulations support the link between these two systems. For example, administration of CRH increases catecholaminergic activity and norepinephrine (NE) levels, while CRH antagonists cause reduced responsiveness in the LC-NE system (15); moreover, NE stimulates the release of CRH in the PVN, while β-adrenergic blockers reduce the behavioral consequences of CRH.
Despite these connections, the two systems may be differently activated by different kinds of stressors and may differently respond to stress over time. Some research suggests that CRH pathways function differently in responding to metabolic versus psychological stressors; thus CRH may affect the autonomic nervous system under conditions of metabolic but not psychological stress (16). Moreover, the HPA axis may be particularly sensitive to fear and frustration, whereas the autonomic nervous system may be more generally responsive (17-21). Since the SNS system is more responsive than HPA axis to stress induced by parachute jumping, this experiment could indicate that fear and frustration are modest in novice parachutists.
Chronic stress may have different implications for the HPA axis and SNS and may thus affect the degree of symmetry between them. Chronically elevated levels of cortisol and CRH may promote long term changes in the functioning of CRH in response to stress (22). Given connections between CRH and the SNS, these changes may have implications for the relation between the HPA axis and the SNS. Experimental manipulations have revealed that repeated stress leads to asymmetry between the two systems. Some researchers report attenuated responses in the HPA axis to experimental manipulations but not in the LC-NE/SNS (23, 24). Our experiment indicates that not only chronic, but also acute stress can lead to asymmetry between the two systems.
The validity of salivary sAA as an index of sympathetic activity is confirmed by the results of the present experiment. Indeed, the change of salivary sAA is similar to HR modifications. The present experiment confirms also that GSR is a sensitive psycho-physiological index of stress. Since the modification of GSR is related to changes in sympathetic arousal (25), the GSR change is similar to sAA change. The evidence reported in this paper corroborate the validity of GRS as a non-invasive tool in research on responses to stress (26).
This experiment should be repeated in experienced jumpers, so you can analyze the effect of experience on the variables of stress. It would be interesting to know if experience increases or decreases the dissociation between the SNS and the HPA axis. Furthermore, the results of these experiments can be applied to programs of military training and military medicine for effects of stress in the paratroopers.
The responses observed in such an extreme situation could apply in other sports environments. The reactions noted in the present study could occur in water sports (such as freediving) or mountain sports (such as fast climbing). These sports are characterized by brief but intense stress and wide range of environmental conditions. The application of the experimental model utilized in the present study could provide further information regarding the reaction in these sports, in order to also improve the training methods.