1. Background
During exercise, there is a regulatory thermal balance between heat production and heat loss, requiring activation of the mechanisms responsible for thermoregulation, which are mediated by a complex feedback hypothalamic system (1). The metabolic heat loss occurs in the following four ways: conduction, radiation, convection, and evaporation (1-3). Depending on the weather conditions, evaporation is the main form of heat dissipation with the production of eccrine sweat (4). When sweat evaporates, there is generally a reduction in the skin temperature (TSK) if the maximum evaporative power of the environment is not exceeded (2).
Another important physiological response during exercise is the regulation of blood flow. Regions of exercised muscles (active) require vasodilatation to increase their oxygen supply, while vasoconstriction occurs in the inactive muscles, reducing the blood flow and TSK supplying the inactive muscles (5-7). The cardiovascular response, involving thermal control and exercise, has not been thoroughly characterised and comprises multiple complex interactions (5, 8). Skin blood flow adjustments to various sites are mainly accomplished through the interaction of both the central and peripheral circulatory mechanisms driving vasodilatation and vasoconstriction (5).
Human cutaneous circulation is highly variable, varying from almost zero L/min, in local or whole body cooling conditions, up to 8 L/min under heat stress conditions (4). The cutaneous blood flow correlates directly with the TSK values recorded by infrared thermography (IRT) (9). IRT does not require contact with the skin, and it is a convenient, reliable and non-invasive technique that has been previously used for monitoring the TSK in a wide range of physical activities (10). IRT quantitatively registers a series of thermal images and can precisely determine the temperature of a specific region of interest (ROI) (local analysis) (10). IRT also allows for the accurate tracking of the thermoregulatory process in a limb or the whole body during various exercise intensities. Therefore, IRT allows us to quantify and map the temperature of a wide body surface, which is impossible to achieve with thermocouples or any other method for registering the TSK.
Another important feature of IRT is the absence of physical contact with the subject, allowing for normal movements during exercise and providing an assessment of the considered ROI without interfering with the natural processes of evaporation, convection and radiation (10). Importantly, IRT measures the direct heat radiated by the skin, whereas thermocouplers are indirectly adjusted from the temperature values transmitted by conduction (10, 11). Some studies have been published that quantify the changes in the TSK during exercise using IRT (7, 12-16). However, in most of these studies, the exercise duration was less than 30 minutes, which is a relatively short effort (7, 13, 14, 16, 17). Moreover, the few available reviews have shown only a limited qualitative analysis (12-14) or have only focused on sparse evaluations of the skin sites (14-16). Under these limitations, our study added as direct variables: extent of exercise time, post-exercise IRT measurement and monitoring largest ROIs.
2. Objectives
Considering the limitations presented in past studies, this study aimed to investigate the TSK variations using IRT in a group of physically active, young people under controlled environmental conditions 30 minutes before exercise, during 60 minutes of intense aerobic exercise and during the following recovery phase. We hypothesized that most ROIs will show differences at the temperatures measured during and after exercise.
3. Patients and Methods
3.1. Participants
The sample consisted of 12 physically active males (age: 22.4 ± 3.3 years, height: 177.0 ± 0.8 cm, percentage body fat: 10.3 ± 3.0%, body surface area: 1.92 ± 0.09 m2 and VO2max: 48.7 ± 4.9 mL.min-1.kg-1). The sample size was determined with the software GPower 3.13, selecting ANOVA with repeated measures for the analysis and considering a size effect (power) of 0.97. The volunteers were informed about the procedures for all stages of the investigation and signed informed consent forms prior to enrolment in the study. This study was approved by the local Ethics Committee on Human Research (No 134, 2011) and the participation was consented in accordance with Helsinki’s declaration.
3.2. Pre-Experimental Procedures
Because external and internal factors can interfere with TSK recordings, the following characteristics were used as exclusion criteria: smoking; history of kidney problems; injury; osteomyoarticular problems or symptoms in the last two months; skin burns, regardless of how the body areas were evaluated; symptoms of pain in any body region; sleep disorders; fever in the last seven days; physical therapy treatments; use of dermatological creams, ointments or lotions; and usage of medications, such as antipyretics or diuretics, or any dietary supplement that could potentially interfere with water homeostasis and body temperature in the last two weeks. The subjects were considered physically active subjects according to the criteria of the American College of Sports Medicine (ACSM); the subjects performed regular physical training sessions at least 3 times a week for four months.
Two days before the experiment day, the body mass in grams (Filizola®, Star 300/4), height in centimetres (American Medical®, ES2020) and skinfolds in millimetres (Cescorf®, Scientific) were measured. The body density was estimated using the equation of the sum of seven folds (pectoral, subscapular, midaxillary, triceps, suprailiac, abdomen and thigh) developed by Jackson and Pollock (18).
The maximal oxygen uptake (VO2max) was estimated using a submaximal incremental treadmill test according to the recommendations of the ACSM (19). In the methodology proposed by the ACSM, the individual equations for estimating the VO2max were formulated by linear regression using the values of the heart rate (HR) in beats per minute (bpm) and VO2 values (mL.min-1.kg-1) obtained during exercise. A metabolic gas analyser (Medical Graphics Corporation®, VO2000) was used to evaluate the oxygen uptake, a heart monitor (Polar Team2 Pro®) was used to measure the HR, and software (SigmaPlot®, 12.0) was used to perform the linear regressions. A researcher who was not involved in data collection performed the previous experimental procedures.
3.3. Procedures During The Experimental Day
Between 8:00 to 8:30 AM, while at home, subjects ingested a thermal pill for evaluating their core temperature (TC) with a telemetry system (HQ CorTemp® Inc., HT150002) (20). Each pill was properly calibrated and certified by the manufacturer.
From 11:00 to 12:00 hours, the subjects consumed a lunch consisting of foods commonly consumed in their daily routine. To avoid physical and thermal stress, the volunteers were transported by car to the laboratory. The volunteers entered the laboratory by 13:30 hours and adapted to room temperature for one hour. This room was properly equipped with artificial fluorescent lamps, and the environmental temperature was maintained through a heating/cooling air conditioner (Komeco®, Hi-wall Split). The average temperature remained at 24.9 ± 0.6˚C, and the relative humidity was 62.3 ± 5.7%; both measures were recorded with a digital weather station and anemometer (Instrutherm®, AD-250), which characterised the environment as temperate with null (≅ 0.2 m/s) wind speed. The HR was monitored at all stages of the study using a heart monitor (Polar Team2 Pro®).
During data collection, the subjects were dressed in training shoes, swimming trunks, and a heart-rate monitor belt. Immediately upon arrival in the laboratory (approximately one hour before starting the data collection), each subject drank 500 mL of water to avoid dehydration during exercise. The TC and HR were continuously monitored, and the data were analysed as the average of the last minute for each specific period of each experiment.
3.4. Pre-Exercise Phase
Because body temperature varies with the time of day, TSK data collection always began at 14:30. The equation proposed by Nadel et al. (21) was used to calculate the mean weighted skin temperature (MTSK = 0.21 × Tforehead + 0.1 × Tchest + 0.17 × Tabdomen + 0.11 × Tscapula + 0.12 × Tarm + 0.06 × Tforearm + 0.15 × Tthigh + 0.08 × Tleg).
The subjects remained standing for 30 minutes in the test room, and one IRT scan was recorded every 5 minutes, totalling seven collections during the resting condition. During the latter period, the volunteer remained in an anatomical position in front of the imager at a distance of 3 meters for the measurements of two thermogram images (anterior and posterior regions of the body). An imager (Flir®, T420) with an accuracy of 2%, sensitivity ≤ 0.05˚C, auto-focusing and a resolution of 320 × 240 pixels was used to obtain the thermograms.
The subjects were weighed to determine the body mass before and after the experiment, and the urine specific gravity was determined with a refractometer (LF®, 107/3) from aliquots of urine collected in 50 mL plastic bottles before and immediately after the start of activity as well as after the experiment to check the level of water loss that was induced by exercise.
3.5. Exercise Phase
In this phase of the experiment, each subject completed a treadmill test consisting of 12 intervals of 5 minutes each with a passive interval of one minute. The intensity of the exercise was determined for each individual based on the calculated 60% of the VO2max speed obtained in the pre-experimental data collection session. HR monitors were used during the test to ensure that the subject was running at the prescribed intensity. During exercise, the IRT images and other variables were recorded at 1 minute intervals.
Dehydration can also affect thermoregulatory responses. Therefore, before starting each exercise block, the subjects ingested 1 mL/kg of body weight of water to ensure hydration. The TC and HR were recorded during exercise at each interval and summed as the average during the last minute of each period.
3.6. Post-Exercise Phase
The subjects remained standing for 60 minutes in the test room, and IRT thermal images were recorded every 5 minutes for a total of 12 times along the entire resting phase. The post-exercise recorders were conducted in the same way as those during pre-exercise for the rest of the variables. During the recovery period, the subjects did not exert any physical effort, did not bathe, did not dry their skin with any type of absorbent material, and did not urinate; the only sweat evaporation during this period occurred naturally. A single researcher performed the data collection.
3.7. Thermal Image Processing
After collecting the entire series of thermograms from each subject, 28 different ROI were established as follows: forehead, face, chest, abdomen, back, lumbar, anterior and posterior neck, and posterior and anterior views of the right and left hands, forearms, upper arms, thighs, and legs. The ROI were defined using specific software (Flir Tools®) with emissivity values adopted for human skin of 0.98 and reflected the room temperature of 25˚C.
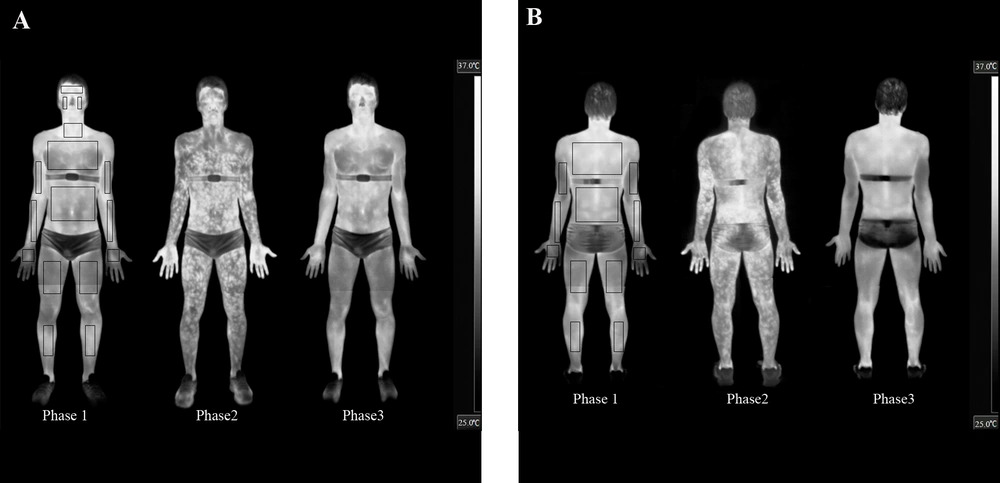
Anatomical reference points were considered for defining the analysed ROI areas (see Figures 1A and B), delimiting the area for identifying the temperatures. A researcher who was not involved of the other stages of the study; performed thermal image processing and statistical analyses.
A, The anterior; and B, posterior views. In every presented moment is possible to observe qualitatively changes the TSK. Note: delimitations of the ROIs are shown in the pre-exercise thermogram only to allow for better discrimination of the thermal effects of exercise on the subjects’ skin.
3.8. Statistical Analysis
For statistical calculations the average of the sum of the TSK recorded in left and right hands, forearms, arms, legs and thighs were considered. The face region was considered as the sum of the cheek and forehead scans.
The Shapiro-Wilk test was used to assess the normality of the data. A one-way ANOVA for repeated measures was used to assess the TSK values as well as for the analysis of the TC, HR and MTSK between different time points. This comparison was followed by the Tukey post hoc test.
The paired t-test was used to compare the variables, such as the body mass and density of the urine pre-and post-exercise. The level of significance was always set as α = 0.05. All analyses were conducted using a statistical program (Sigmaplot®, version 12.0).
4. Results
There was a significant difference (P < 0.01) in the pre-exercise body mass (73.8 ± 6.3 kg) compared to post-exercise (73.3 ± 6.2 kg). The pre- (1014.1 ± 5.6) and post-exercise (1013.1 ± 5.8) urine specific gravity values were not significantly different (P = 0.46). Figures 1A and B show the thermal response of the skin after 60 minutes of aerobic training and after 60 minutes of recovery for one of our subjects.
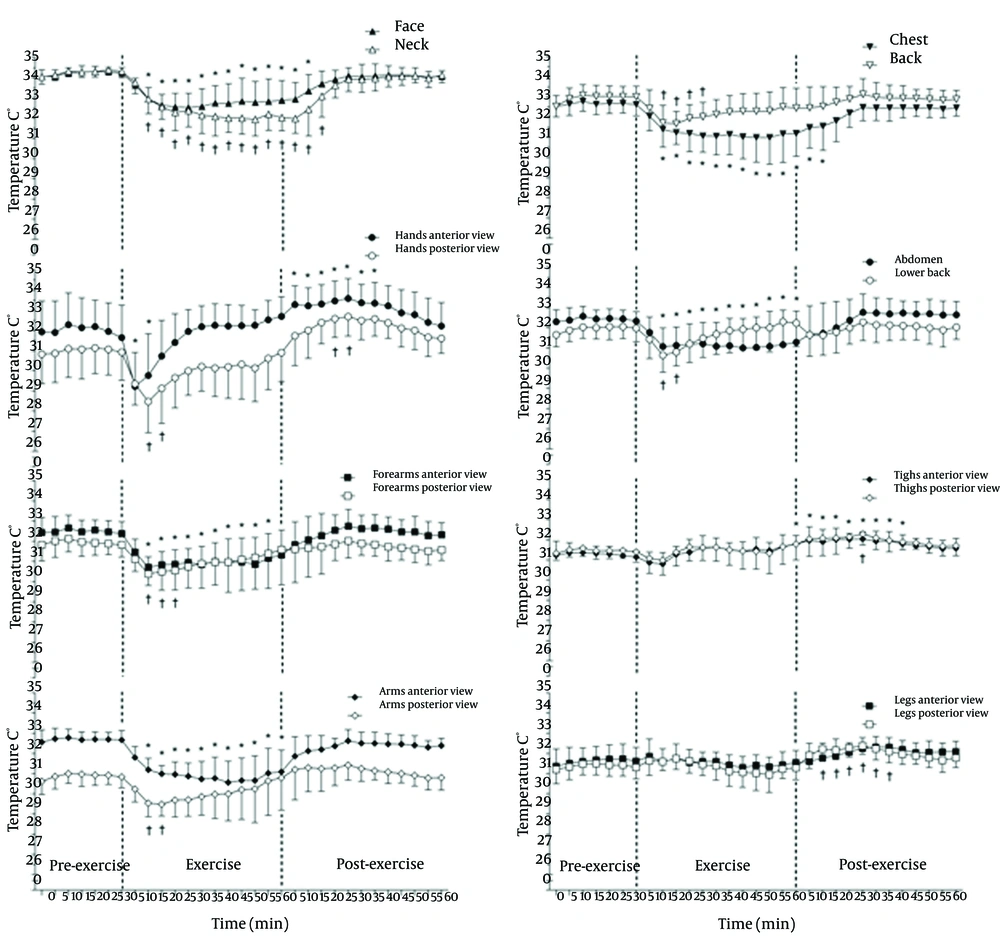
Figure 2 shows the pre-exercise, exercise and post-exercise TSK values of the different ROI and grouped ROI in the anterior and posterior views. The TSK in the pre-exercise phase is stable, and the TSK values are significantly reduced 10 minutes after the start of exercise (P ≤ 0.05) in most of the considered ROIs, except for the anterior and posterior views of the thighs and legs and the anterior view of the hands, which is reduced within 5 minutes of the beginning of exercise. The significant reduction in the TSK (P ≤ 0.05) remains after the completion of exercise in the anterior forearms, anterior abdomen and anterior arms or until 10 - 15 minutes of recovery in the face, neck and chest; however, this reduction is maintained only for 10-15 minutes for the anterior and posterior hand, the posterior arms and lower back and for 20 - 25 minutes on the back and posterior forearms. There was a completely different thermal response to exercise in the lower limbs without a significant decrement during the 60 minutes of exercise and with significant increments in the TSK (P ≤ 0.05) in the anterior thighs and posterior legs, which were not maintained at the end of the 60 minutes of recovery.
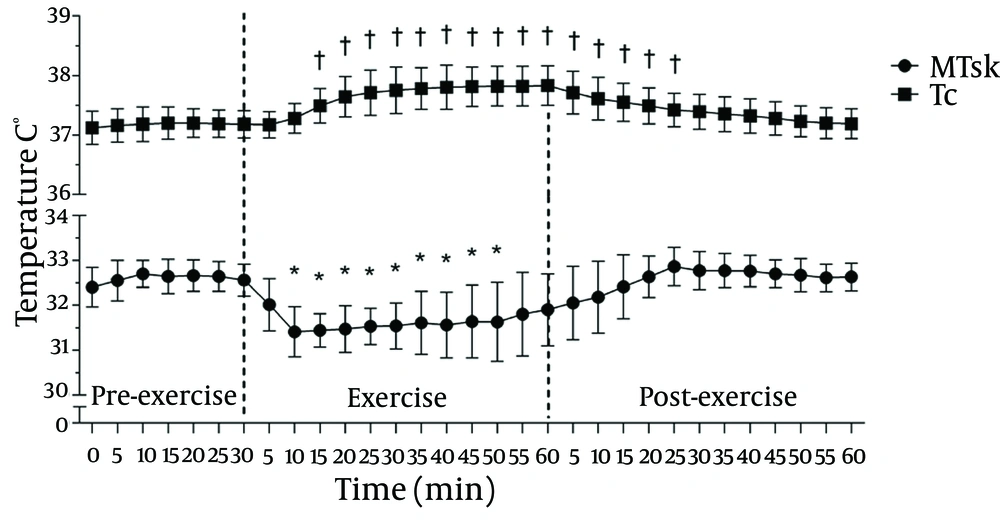
Figure 3 summarises the evolution of the MTSK and TC values with time during the pre-exercise, 60 minutes of exercise, and post-exercise phases. There was a clear reduction in the MTSK response (P ≤ 0.01) up to 50 minutes after exercise; on the contrary, the TC shows a typical ascending rise (to approximately 37.7˚C, which is based on the relative work load) during exercise and a gradual downward trend after its completion.
5. Discussion
5.1. Analysis of the Pre-Exercise Phase
Our data show a consistent temperature pattern and stability in the TSK values of all of the considered ROIs during the pre-exercise phases, which is most likely due to the very long acclimatisation period of one hour in the experiment room before starting the data collection. On that point, Hart and Owens (22) observed a constant decrease over 31 minutes of acclimatisation, with stabilised patterns after 16 minutes, and the typical time required to reach an adequate stability in the Tsk is approximately 10’ (23).
In the pre-exercise control phase, the highest TSK values were recorded in the neck region (34.3 ± 0.3˚C) and face (34.2 ± 0.2˚C). TSK values were observed in the central region of the body (trunk), arms and forearms were higher in the anterior view versus posterior view. The lower limb ROIs (thighs and legs) had lower TSK values. A possible explanation for these results is that during the resting condition, most of the heat produced by the body originates via heat flux from the major organs that are located in this central region, sequestering most of the blood flow away from these skin sites (3).
5.2. Analysis of the Exercise Phase
With the beginning of exercise, there are important changes in the TSK compared with the resting baseline. Reduction in the TSK was observed in many of the studied ROIs until different times following exercise completion, except for the thighs and legs in the anterior and posterior views. These results reinforce the concepts of the redistribution of blood flow in the region of the skin corresponding to the active muscles (5, 6, 8). Similar results were observed in previous studies (12-17).
The most significant reductions occurred in the hands (posterior view: 2.5˚C; 7.9% and anterior view: 2.4˚C; 7.7%), followed by the forearms, arms (both views), pectoral, abdomen, back and lumbar. However, the reductions are maintained for a shorter period of time in the hands and posterior forearms, arms, back and lower back. These results confirm that TSK exhibits variable temperature patterns due to the redirection of cutaneous blood flow to these ROIs, facilitating heat loss to the environment (6, 14, 24). In some recent studies, the sweating rates (from more sweat glands) were reported to be highest in the back and lumbar regions during exercise (25).
This finding can also be explained by greater stimulation of the cutaneous vasoconstrictor responses that are controlled by adrenergic nerves of the sympathetic system and most likely modulated by the action of noradrenergic neurotransmitters, such as norepinephrine and/or neuropeptide Y (4, 5, 8, 26). These factors possibly redistribute the blood flow in these regions from inactive skin to the muscles.
Considering the whole body values, there was a statistically significant reduction in the MTSK values (P ≤ 0.01) after 10 minutes of exercise, with a magnitude drop of 1.2˚C (3.5%). Similar results were observed in another study (27) using thermocouplers for measuring the TSK. Regardless of the method of assessment, it is possible to observe a reduction in the TSK in non-active regions during exercise due to the action of cutaneous vasoconstriction and flow redistribution.
It is intriguing that the TSK observed from the more active regions (thighs and legs) did not register significant differences at the initiation of the exercise that were obtained for the other considered ROIs. Our subjects were very well-trained individuals; therefore, the action of vasoconstrictor mechanisms on these regions under mild exercise conditions may not have produced enough of a physiologic strain with the metabolic activity of the muscles involved in running. Apparently, the redistribution of blood flow during exercise is compensated by thermal conduction to specific skin areas. It appears that a thermal steady-state is reached; optimal skin blood flow in exercise-trained individuals promotes a subsequent prime condition for maintaining the muscular activity as operational during exercise with limited fatigue. This pattern may not occur in unfit, untrained individuals. Additionally, in fit individuals, β-Adrenergic receptor activity is thought to mediate the exercise-induced peripheral cardioprotective effects on the skeleton muscle (28). These latter two mechanisms are not entirely facilitated in untrained or physically unfit individuals.
The IRT technique used in this study shows that it is possible to quantify and characterise the patterns of changes in the TSK during exercise in different ROIs, focusing attention on the variation in the TSK within the same ROI, which shows marked increases with exercise completion (Figures 1A and B). Merla et al. (7) found many areas with high concentrations of hyperthermic “tree-shaped” points that branch into smaller areas that maintain this elevated temperature. A similar situation was also evident in the present study (see Figures 1A and B) and may correspond with the perforator vessels that provide blood flow to the different levels of the skin structures (29). Hunold et al. (13) also reported that within the distance of several centimetres, the TSK differences were above 3˚C and the differences in the microcirculation of the skin were up to 300%.
5.3. Analysis of the Recovery Phase
At the end of the exercise, the TSK remained stable in the back, abdomen, lower back, forearms and arms in the anterior and posterior views and in the anterior view of the legs. However, in the anterior and posterior views of the hands and thighs and in the posterior legs, there were recorded increases in the TSK. Thus, there was a significant change in the distribution patterns of the TSK, where the tree-shaped hyperthermic points that appeared during exercise were more widespread and uniform within the ROIs after exercise. One possible explanation for the increases recorded in the TSK may be due to a decrease in the vasoconstrictor activity and increased vasodilatory activity through the action of nitric oxide, acetylcholine, VIP and substance P (5, 6), thereby increasing blood flow to the skin in order to increase the heat dissipation to the environment re-establishing the normal conditions of TC that were above resting values after 25 minutes of recovery. The large increases in the TSK persisted in some cases for up to 40 minutes after exercise, indicating that the return of the TSK values to the resting values is relatively long for certain ROIs and that each ROI responds differently to the end of aerobic exercise.
Significant increases in the TSK observed in the lower limbs after exercise can be explained by the increased blood flow in active muscles (5, 6). When exercise is completed, there is a predominance of cutaneous vasodilator mechanisms operating with the sympathetic drive (operating even after the gradual core temperature decline after exercise) (7).
Another physiological response that deserves attention is the post-exercise behaviour of the TSK in the regions of the palms (glabrous skin) and dorsal hands (non-glabrous). Yamazaki (24), using laser-Doppler flowmetry, reported that the blood flow of the glabrous skin of the palms is 3.2 times higher and that of the non-glabrous skin of the dorsal hand during sitting rest and up to 7.4 times higher during exercise. Furthermore, the palms receive only vasoconstrictor innervation, and the dorsal hands have vasodilator and vasoconstrictor nerves (5, 26). Therefore, the lowest TSK values in the dorsal region of the hand after exercise (compared with the palmar region) may be due to the different blood flow supply to the specific areas or to the higher (or more effective) control of the vasodilator mechanisms in the dorsal hands, which is not as intense in the palms (5).
IRT allowed us to globally map the TSK (Figures 1A and B) or segment it into various ROIs (Figure 2), obtaining important qualitative and quantitative information during rest, exercise and post-exercise conditions in a temperate environment.
Among the limitations of the study, we highlight the lack of control group, quantification of sweating in any of the sites studied and the an association with sweating plotted as a function of the thermal drive, which has been previously documented (9). Additionally, the baseline wind speed was nominal; therefore, our study is limited by not simulating the action of headwind effects to facilitate heat loss through convection.
In this study, we present new findings quantifying the skin temperature variability with IRT before, during and after moderate exercise. Our results demonstrate significant distinctions in the skin temperature distribution during exercise according to the activity of the considered area during exercise, which may be important in the development of physiological models and heat flux analyses for different purposes.