1. Background
Energy production is a necessary process to continue physical activities, but energy production leads to the generation of oxidative stress (1-3). Thus, exercise is associated with more oxygen consumption and increase of the oxidative stress (4-6). Oxidative stress includes definitions that have undergone some changes with the development of science. However oxidative stress reflects the imbalance between antioxidant activity and free radicals (7-9). Studies have indicated that the activity of antioxidants is influenced by the amount of free radicals that are produced during physical activity, also gender has effect on this antioxidant response (10-13). It has been reported that estrogen in women leads to increase of antioxidant enzymes activity in the immune system and other cells through estrogen receptors (14, 15). The estrogen receptors are divided into ERa and ERb, that ERb receptors are more predominant in lymphocytes and by which the activity and gene expression of antioxidant enzymes are more affected (16-18).
But what seems important is the numerical relationship between antioxidant and free radicals. Although the activity of some enzymes increases with physical activities, but it is possible that gene expression of the this enzyme is not changed during exercise (11, 19). Some studies suggest the increased activity of antioxidant enzymes during intense physical activities (20), but the activity of an enzyme does not necessarily mean the associated gene expression. Also, it is not clear that to what extent changes in the level of free radicals impact MnSOD, Cu/ZnSOD and catalase enzymes expression and also total antioxidant capacity (TAC) and how gender can affect this process. Therefore, it seems that physical fitness levels and gender can produce different responses in terms of gene expression and activity of antioxidant enzymes (20-22). On the other hand, the quantitative (numerical) relationship between gene expression of antioxidant enzymes and their activity associated with increased level of free radicals is not defined. As previous studies have not reported whether the relationship is direct (positive) or indirect (negative), so it is not clearly defined that for every one unit increase in the level of MDA, how gene expression and activity of antioxidant enzymes changes and how gender affects the relationship.
2. Objectives
Thus, the aim of the present study was to examine the gene expression of mitochondrial and cytosolic antioxidant enzymes and changes in MDA and TAC level in active men and women affected by an incremental exercise session and to carefully and numerically investigate the relationship between changes in MDA and gene expression and activities of antioxidant enzymes.
3. Materials and Methods
3.1. Subjects
Twenty four active and healthy men and women (aged 21 - 24 years old, BMI: 22 - 25 Kg/m2) volunteered in this study. All volunteers attended the exercise venue and received information about the protocols and procedures, as well as the possible risks and benefits involved in the study. Informed written consent was obtained from all the participants. The ethics committee of Tabriz University of Medical Sciences, Iran, approved the study design. To determine their inclusion in the study, volunteers completed medical history and physical activity questionnaires (PAS) (23), and took part in physical examinations. The volunteers also were advised to avoid the use of caffeine, nicotine, alcohol, antihypertensive medications, and an exhaustive workout (which could influence the immune response) in the previous hours. The subjects were then randomly assigned to the men (n = 12) and women (n = 12) groups.
3.2. Measurements
Each volunteer was asked to empty their bladder before laboratory examinations and tests. In basal state we measured age, height, weight, heart rate (HR), fat %, body mass index (BMI), maximal oxygen uptake (VO2max) and lipid profile. Gene expression of MnSOD, Cu/ZnSOD, catalase enzyme and TAC, MDA levels measured in three stages; pre exercise test, immediately and 3 hours after exercise (recovery). Body weight (kg) and height (cm) were measured using the standard digital floor scale and precision stadiometer (Seca, Germany.) Body fat percent and BMI (kg/m2) were measured using the portable bioelectrical impedance system (body composition, Omron, China). Maximal oxygen uptake (VO2max, mL.kg-1.min-1) was measured by performing a test on an electronically braked bicycle ergometer (Monark 839 E, Sweden), with initial resistance set at 60 W and 20 W step-wise increments every 3 minutes. Heart rate and rhythm were continuously recorded by 12-lead electrocardiogram and subjects reported their rate of perceived exertion (RPE) on the 15-point Borg scale at the end of each stage (Noble et al. 1983). The volume of oxygen consumed (V O2) and carbon dioxide produced (V˙CO2) during exercise were calculated from minute ventilation (V), measured using mass flow ventilometry, and simultaneous mixing chamber analysis of expired gas fractions (V max E, Sensor Medics, Yorba Linda, CA). Gas analyzers and flow probes were calibrated before each test. V˙O2 and V˙CO2 were recorded during the final 40 seconds of each stage of the test and at peak exercise and expressed in L min-1 and also relative to body weight (mL.kg-1.min-1) (Andrew et al. 2001). The fix amount of food for volunteers was determined and all volunteers received a detailed verbal explanation and written instructions. Any questions regarding the type and amount of food and beverages consumed were resolved individually (24).
3.3. Exercise Protocol
Subjects started walking on the treadmill (EXE T600, European exercise equipment, EC, TUV) for 3 minutes (Grad: 0%, Speed: 3 Km.h-1) According to the graduated exercise test (GXT). Then up to 5 minutes, g rad of the treadmill was increased to 5% and then did not change. But treadmill speed was increased to 13 Km.h-1 until the end of the training period, and at the end of the training, volume and intensity was increased to 25 minutes and 80% of maximal exercise heart rate. The exercise training was performed at the ambient temperature, 23 - 24°C and relative humidity of 42%. Subjects were supervised by trained physical education teachers. All exercise sessions began between 09.00 and 11.00 hours to avoid circadian rhythm effects.
3.4. RNA
For RNA extraction and biochemical assays, 4 mL blood samples, following a 12-hour overnight fast, were taken between 08.00 and 09.00 hours. Samples were taken at baseline, immediately and 3 hours after exercise. All samples were taken 24 hours after the last exercise bout. Total RNA extraction from peripheral blood (lymphocyte cell) was performed using RNX-PLUS reagent (Cinagen, Iran) following the manufacturer’s protocol. Clean-up procedure was carried out to avoid any genomic DNA contamination during qRT-C with a specific wipeout buffer (Quanti Tect Reverse Transcription, Qiagen, Germantown, USA). The RNA quantity and quality were assessed by spectrophotometry. The reverse transcription reaction was performed using revert aid first standard cDNA kit (Fermentas). For cDNA synthesis, RNA was treated with DNaseI (Invitrogen) and a 20 Rl reaction solution containing: 4Rl 5X reaction buffer, 1 Rl RNase inhibitor (20u/Rl), 2 Rl dNTP mix (10mM), 1 Rl random hexamer primer, 1 Rl oligo (dt) 18 primer, and placed 5 minutes at 65°C and 60 minutes at 42°C. Reverse transcription activity was terminated by heating at 70°C for 5 minutes. For RNA extraction 1 mL of RNX solution was mixed with 5 × 106 cells and incubated at room temperature for 5 minutes. After adding 200 Rl chloroform it was centrifuged at 12000 RPM at 40 3 C for 15 minutes and then transferred to a tube containing an equal volume of isopropanol. After centrifugation at 12000 RPM, 40 C, 15 minutes the supernatant was discarded and mixed with 1 mL of 75% ethanol. Then the pellet was dissolved and centrifuged for 8 minutes at 7500 RPM. Finally the supernatant was discarded and the pellet after drying was dissolved in 20 Rl DEPC-treated water. Approximately 2 Rl of RNA were used to determine RNA yield and quality by optical density reading at 260 nm and 280 nm. Finally, for each sample, real-time PCR reactions were carried out: 20 Rl volume/well containing 10 Rl of 2 × master mix (10 Rl of 2 × SYBER Green PCR master mix reagent kit (ABI, USA), 0.3 Rl primer, 2 Rl cDNA and 7.4 Rl DEPC-water mixture) for the tested gene and β-actin RNA. Then the plate placed into the thermocycler (Corbett-gene 6000). The thermal cycle conditions were 95°C for 10 minutes followed by 40 cycles that were run for 15 seconds at 95°C (denaturation) and for 35 seconds at 600 C (Annealing). Expression of MnSOD, Cu/ZnSOD and catalase were standardized with β-actin RNA as endogenous control by melting curve analysis and by 1% agarose gel electrophoresis (Salemi et al. 2011). The primers used for the MnSOD cDNA amplification were F-5’-GAGAAGTACCAGGAGGCGTTG-3 and R-5’-CAAGCCAACCCCAACCTGAGC-3’, for Cu/ZnSOD F-5’-AAGGCCGTGTGCGTGCTGAA-3 and R-5’-CAAGTCTCCAACATGCCTCT-3’ and for catalase F-5’-TTTGGCTACTTTGAGGTCAC-3 and R-5’-TCCCCATTTGCATTAACCAG-3’. The primers used for β-actin were F-5’-CAGGTCATCACCATTGGCAAT-3’ and R-5’-TCTTTGCGGATGTCCACGT-3. To estimate mRNA expression levels, Real-time PCR reaction efficiency was calculated using the standard curve slope. Standard curve drawing was based on serial dilution of the genes of interest against the logarithm concentration of template DNA. MnSOD, Cu/ZnSOD and catalase and β-actin RNA efficiencies were 98% and 97%.
3.5. MDA
Plasma MDA levels were determined by using the methods of Draper and Hadley based on thiobarbituric acid (TBA) reactivity (25).
3.6. TAC
The total antioxidant capacity of plasma (TAC) to scavenge ABTS radicals was measured by a chromogenic method with commercially available kit (Cat. No. NX 2332, Randox, Crumlin, UK) (26).
3.7. Lipid Profile
Total cholesterol, TG, LDL-cholesterol and HDL-cholesterol were determined by enzymatic method (27).
3.8. Statistical Analysis
Kolmogorov-Smirnov test was used to check the normality of data, based on these results the appropriate test was chosen. To compare the values between active and women, we employed the t-test (or Mann-Whitney). The effect of exercise on different parameter intra group was evaluated by repeated measure and Bonferroni test. Also, we used linear mixed model for the relation between groups. All statistical analysis was performed using SPSS 22 (Chicago, IL- USA). P values less than 0.05 were considered as statistically significant.
4. Results
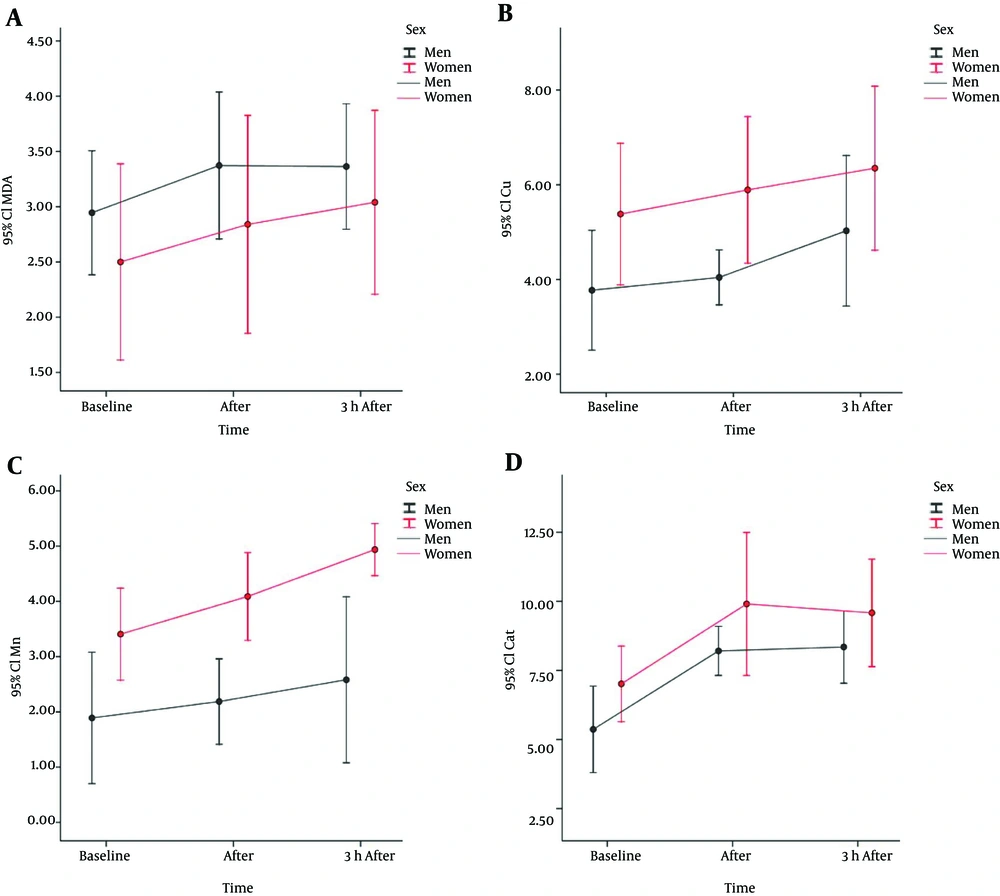
Physiological markers and lipid profile of active men and women have been identified in Table 1. Statistical analysis indicated that gene expression of MnSOD enzyme in active men had increased as a result of incremental physical activities and the increase was also observed in the recovery phase, however, these changes were not significant (P = 0.99). Statistical analysis on gene expression of Cu/ZnSOD enzyme in active men showed that the enzyme mRNA levels have increased immediately after exercise and in the recovery phase, but the changes were not significant (P = 0.346 and P = 0.99, respectively). However, catalase gene expression in active men significantly increased immediately after exercise (P = 0.005). The changes also continued to the recovery phase and catalase mRNA level increased significantly (P = 0.003). For total antioxidant capacity (TAC), our studies showed that TAC levels have increased immediately following exercise and also 3 hours later (during recovery), in active men. However, only changes in the recovery phase (3 hours after exercise) were significant (P = 0.437 and P = 0.009, respectively) (Figure 1).
| Variables | Active Men | Active Women | Pa |
|---|---|---|---|
| Age, y | 23 ± 1 | 21 ± 1 | 0.168 |
| Height, cm | 177.59 ± 7.02 | 160.8 ± 4.35 | 0.329 |
| Weight, Kg | 70.54 ± 6.1 | 53.1 ± 5.78 | 0.947 |
| Vo2max, mL/kg/min | 55.97 ± 1.35 | 52.6 ± 1.83 | 0.713 |
| Body mass index, kg/m2 | 22.5 ± 1.5 | 25.6 ± 1.83 | 0.122 |
| Heart rate in basal state, beat/min | 78 ± 7.52 | 67.3 ± 8.67 | 0.766 |
| TG, mg/dL | 86 ± 36.65 | 75.3 ± 18.97 | 0.701 |
| Cholesterol, mg/dL | 131 ± 14.24 | 134.3 ± 22.72 | 0.708 |
| HDL-C, mg/dL | 35.11 ± 5.49 | 46 ± 10.02 | 0.821 |
| LDL-C, mg/dL | 102.10 ± 5.12 | 96.60 ± 4.26 | 0.512 |
aBased on independent t test (P ≤ 0.05).
Furthermore, repeated measures analysis and post hoc Bonferroni test revealed that MDA levels have significantly increased immediately after exercise in men, but in spite of a slight decrease in the recovery phase, the levels were still above the ground state and the difference was significant (P = 0.012 and P = 0.014, respectively) (Figure 1).
Furthermore, mixed model and linear regression analysis showed there was a reverse relationship between changes in MnSOD and Cu/ZnSOD levels and MDA in men (P = 0.001 and P = 0.05, respectively). So that for every one unit increase in the level of MDA, there was a decrease of -3.69 and -2.62 units in gene expression of MnSOD and Cu/ZnSOD, respectively. However, there was not a significant relationship between gene expression of catalase and TAC and MDA in active men (P = 0.456 and P = 939, respectively). As per one unit increase in the level of MDA, catalase gene expression and TAC levels decreased 0.93 and 0.01 units, respectively (Table 2).
| Response | Variable | Active Men | Active Women | ||
|---|---|---|---|---|---|
| B (95% CI) | P | B (95% CI) | Pa | ||
| Cu/znSOD | MDA | -2.62 (-5.26 to 0.02) | 0.050 | 2.9 (0.66 to 5.14) | 0.014 |
| MnSOD | MDA | -3.69 (-5.57 to -1.8) | 0.001 | -0.14 (-1.5 to 1.23) | 0.836 |
| Catalase | MDA | -0.93 (-3.57 to 1.71) | 0.456 | 0.16 (-2.46 to 2.77) | 0.901 |
| TAC | MDA | -0.01 | 0.939 | 0.11 (0 to 0.22) | 0.051 |
aBased on mixed model statical analysis (P ≤ 0.05).
The statistical analysis in the group of active women showed that there has not been a significant increase in MnSOD gene expression immediately after exercise (P = 0.421). Yet gene expression of the enzyme increased significantly in the recovery phase (P = 0.006). For the changes in Cu/ZnSOD our studies also showed that gene expression of the enzyme in women has not significantly increased immediately after exercise and during recovery (P = 0.99). Catalase gene expression in active women significantly increased immediately after exercise (P = 0.03), but decreased three hours later and in the recovery phase and was not significantly different from the ground state (P = 0.06). Statistical analysis using the Bonferroni test also showed that TAC level in women has increased significantly immediately after exercise (P = 0.031), but decreased slightly in the recovery phase (P = 0.065) (Table 2).
The MDA concentration was not significantly increased immediately after exercise in women (P = 0.255), but 3 hours after exercise, level of this blood index increased significantly (P = 0.029) (Table 2). Statistical analysis using mixed model test and regression analysis showed that there is not a significant relationship between changes in MDA and gene expression of MnSOD and catalase (P = 0.836 and P = 0.901, respectively), in active women. As per one unit increase in MDA level, the gene expression of MnSOD decreased -0.14 units and catalase level increased 0.16 units. However, the relationship between changes in MDA and gene expression of Cu/ZnSOD and TAC was significant (P = 0.014 and P = 0.004, respectively). As per one unit increase in MDA level, the gene expression of Cu/ZnSOD and TAC increased 2.9 and 0.11 units, respectively (Table 2).
Further statistical analysis showed that in all phases of the study (baseline, immediately after exercise and three hours later) active women had higher levels of gene expression of antioxidant enzymes and also TAC. However, in the case of Cu/ZnSOD in the phase immediately after exercise, difference between the two groups was P = 0.017, and for MnSOD difference between the two groups was P = 0.033, P = 0.001 and P = 0.005 at all stages, the difference was reported significant. However, no significant difference was observed in the case of catalase and TAC (Table 3).
| Var | Men | Women | Between Group P | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Cu/Zn | |||||
| Baseline | 3.77 ± 1.88 | 4.54 (0.5 to 5.62 ) | 5.38 ± 2.09 | 5.55 (0.33 to 7.72 ) | 0.079a |
| After | 4.04 ± 0.86 | 3.87 (3.17 to 6.27 ) | 5.89 ± 2.17 | 6.73 (2.45 to 9.08 ) | 0.017a |
| 3 hours after | 5.03 ± 2.37 | 4.8 (0.4 to 8.79 ) | 6.35 ± 2.42 | 6.08 (3.55 to 10.47 ) | 0.222a |
| Mn | |||||
| Baseline | 1.89 ± 1.77 | 1.29 (0.02 to 5.99 ) | 3.41 ± 1.16 | 3.36 (1.77 to 6.25 ) | 0.033a |
| After | 2.19 ± 1.15 | 1.91 (0.86 to 4.15 ) | 4.09 ± 1.11 | 3.89 (2.78 to 5.86 ) | 0.001a |
| 3 hours after | 2.58 ± 2.24 | 1.82 (0.41 to 8.3 ) | 4.94 ± 0.66 | 5.22 (3.75 to 5.84 ) | 0.005a |
| Cat | |||||
| Baseline | 5.37 ± 2.33 | 5.43 (1.12 to 9.73 ) | 7.01 ± 1.91 | 6.89 (5.01 to 10.76 ) | 0.095a |
| After | 8.21 ± 1.32 | 7.98 (5.98 to 10.28 ) | 9.91 ± 3.62 | 10.34 (3.35 to 15.71 ) | 0.161a |
| 3 hours after | 8.35 ± 1.95 | 8.17 (5.82 to 13.31 ) | 9.58 ± 2.72 | 8.87 (6.2 to 14.22 ) | 0.242a |
| MDA | |||||
| Baseline | 2.95 ± 0.84 | 3.2 (1.2 to 3.8 ) | 2.5 ± 1.24 | 2.15 (1.2 to 4.2 ) | 0.512b |
| After | 3.37 ± 0.99 | 3.6 (1.7 to 4.6 ) | 2.84 ± 1.38 | 2.75 (1.3 to 4.9 ) | 0.349b |
| 3 hours after | 3.36 ± 0.85 | 3.6 (1.8 to 4.4 ) | 3.04 ± 1.16 | 3.35 (1.4 to 4.3 ) | 0.605b |
aBased on t-test (P ≤ 0.05)
bBased on Mann-Whitney test (P ≤ 0.05)
5. Discussion
Some research studies emphasize that active men compared to active women have higher activity of the electron transport chain and the xanthine oxidase enzyme that leads to the production of high levels of free radicals (28-30). As our statistical findings show, despite significant increase in MDA level in both active men and women as a result of physical activity, men have higher levels of MDA. In this regard, cardiovascular and respiratory system plays an important role (31, 32). Since athletic men have an effective cardiovascular system and also have highly developed capillary and mitochondrial density, thus the production of O2 and the duration of its presence in tissues is very high (28, 33, 34). So, further increase in free radicals in men will not be unexpected. But research has shown that estrogen prevents the increase of cell damage by increasing the activity of antioxidant enzymes (14, 35).
In this context, statistical review of the present study also showed that women compared to men have higher levels of gene expression of antioxidant enzymes and TAC levels. Other studies in this regard show that lymphocytes and other cells of women have estrogen receptors (36, 37). These receptors can be divided into classes of ERa and ERb, of which ERb receptors are more predominant in the cells of the immune system by which the activity of lymphocytes and antioxidant enzymes are more affected (17). The same studies suggest that the gene expression of antioxidant enzymes, including MnSOD and catalase may be influenced by estrogen (14, 38).
In their findings, the researchers reported that in addition to its effective role in the expression of the estrogen receptor gene, estrogen has a direct relationship with gene expression of antioxidant enzymes (17). Therefore, estrogen increases gene expression of antioxidant enzymes by increasing gene expression of ERa receptors, as well (17). Our findings also showed that MnSOD enzyme gene expression had not significantly increased in active men, however, MnSOD gene expression changes were significant in women. These findings are consistent with those obtained by Ademoglu et al. The investigators reported that MnSOD activity is higher in women than in men (39). Therefore, it is likely to indicate the higher influence of estrogen on MnSOD (37). Investigations by Gottipati et al. showed that estrogen leads to a further increase in MnSOD in women (40).
On the other hand, catalase is an enzyme that causes to produce free radicals through reaction with H2O2 and preventing Fenton reaction (41, 42). In our studies, we found that catalase gene expression in both groups of active men and women had a significant increase. In this context, the role of epinephrine seems important. Studies have shown that epinephrine in addition to driving energy mechanisms to maintain the activity of the body, leads to increased NO production through beta-adrenergic receptors (43). However, there is a significant relationship between NO and catalase. It has been recently reported that NO leads to increased activity of catalase in order to deal with H2O2 (44). Thus, it seems that one of the mechanisms to increase catalase in the present study in both groups of men and women, may be related to the increased level of NO in sporting activities.
On the other hand, in the present study gene expression of Cu/ZnSOD did not significantly increase as a result of incremental exercise. These findings are consistent with the research by Sachdev et al. (45). Studies show that active individuals have adapted in part to an increase in free radicals (45, 46). Thus, in this study the level of TAC in both active men and women significantly increased. It also seems that MnSOD prevents initial increase of free radicals to some extent and Cu/ZnSOD enzyme deals with the less challenges to fight free radicals. However, these cannot be accepted with certainty. As the relationship between free radicals and antioxidant enzyme gene expression is also important and should be examined quantitatively.
Our results indicate that increased levels of MDA resulted in a -3.69-unit decrease of MnSOD gene expression in men and a -0.14-unit decrease in women. However, the numerical relationship is different for Cu/Zn SOD and catalase, and TAC. Thus, our findings suggested that there is an inverse relationship between changes in antioxidant parameters and MDA in men and increase in MDA level is associated with a decrease in the Cu/ZnSOD (-2.62 units), catalase (-0.93 units) and TAC (-0.01 units). But there is positive relationship between changes in these parameters and MDA in women. Thus, in line with changes in MDA, gene expression of Cu/Zn SOD (0.051 units), catalase (0.456 units) and TAC (0.11 units) increased, that seems important immunologically. Since the more the response of the immune system is weakened, the more the injuries will be. In our findings despite the fact that gene expression of catalase, Cu/ZnSOD and Mn SOD enzymes and also TAC level increased in both active men and women as a result of incremental physical activity, but regarding the negative or positive relationships between these indicators MDA, it seems that mitochondria and cytosol of lymphocytic cells of men are more susceptible to damage caused by free radicals compared with active women.
5.1. Conclusions
MnSOD gene expression in active women, despite weakening by the increase in MDA, is still an appropriate response to free radicals and partially prevents the spread of the resulting damage. On the other hand, about cytosol protection it is important to note that the immune system of active men will respond to changes in free radicals by increasing catalase. However, in active women the response occurs through increased gene expression of catalase and Cu/ZnSOD. Thus, it appears that the increased free radical during incremental exercise challenges gene expression and activity of antioxidant enzymes. However, despite the negative effects of free radicals on active women, activity and gene expression of antioxidant enzymes is an appropriate response to the free radical and their gene expression and activity increases in line with the increase in MDA.