1. Background
The human body continually produces reactive species derived from oxygen and nitrogen (RONS). For many years, RONS were considered to be harmful to the health due to damages which they cause and because of their involvement in many diseases and aging. However, in our days, this vision has completely changed and oxidative stress is no longer defined as an imbalance between pro-oxidant/antioxidant factors but as “a disruption of redox signaling and a loss of cellular homeostasis control” (1).
In fact, at low rates, RONS can improve the proper cellular function by regulating many transcription factors, modifying certain signaling pathways and the expression of certain genes (2). However, at high levels, RONS can increase lipid, DNA, and protein oxidation. They can also play a major role in cardiovascular dysfunction, impair the immune system, and increase the risk of some cancers (3, 4). This may be due to the adverse effects of the RONS products released, especially during the reaction between free radicals and polyunsaturated fatty acids leading to the formation of several aldehydes namely the malondialdehyde (MDA) (2).
Several studies noted that isolated exercise with sufficient intensity and duration can increase significantly RONS generation disrupting temporarily the redox homeostasis (1-3). However, data regarding effects of chronic exercise are inconsistant. This can be due to many factors such as the level of adaptation (i.e. trained vs. untrained), exercise load (i.e. moderate, submaximal, maximal, supra-maximal) and living conditions of athletes (i.e. alcohol, altitude, hyperthermia, smoking, diet ...) (1). Nevertheless, it was suggested that chronic physical exercise, when practiced rationally, induces an adaptive process involving “up-regulation” of endogenous antioxidant enzymes (5). In fact, RONS produced during exercise act as an intracellular signal activating transcription factors and signaling pathways leading to the expression of the main enzymes of the defense against radical species. For this reason, physical training was considered as a “natural antioxidant”, used as an effective process to prevent and to treat many diseases related to chronic oxidative stress (1, 2, 5).
During many years, mitochondria have been considered as the main source of RONS during prolonged aerobic exercise. Relevant studies suggested that 3% - 5 % of the total oxygen consumed by athletes may produce superoxide (1, 2, 6). Some other tissues, such as the heart and the white blood cells, may contribute to the total body production of RONS (7). Many other factors are known also as being able to increase free radical generation in the intra and extracellular space, such as NAD (P) H oxidase enzymes (8), xanthine oxidase activation (9), and catecholamine oxidation (10). These factors might be operative to a greater extent during strenuous anaerobic exercise (1, 5). The inflammatory process that accompanies muscle damage can also produce a significant amount of superoxide in an effort to protect against invading organisms (7). The degree of the muscle damage and the intensity of exercise are important factors that promote the release of pro-inflammatory markers such as interleukin-6 (IL-6) (11) and tumor necrosis factor alpha (TNF-α) (12).
In fact, several similarities were noted between the systemic inflammatory response to strenuous physical exercise, sepsis and trauma. Nevertheless, no significant relationship was recorded between the rise of IL-6 (~50-fold or more) and that of TNF-α which is small but significant (11). According to Kimura et al. (12), the exercise characteristics (i.e. intensity and volume) and the involved muscle mass may influence the release of these cytokines in blood. Interestingly, a significant correlation was noted after intense and prolonged physical exercise between IL-6 concentration and muscle damage biomarkers (13). Numerous relevant studies suggested also that IL-6 has an anti-inflammatory effect, acts as a sensor in the glucose metabolism, exerts an effect on fatty tissue and increases the lipolysis process notably during prolonged aerobic exercise (11, 13, 14).
As mentioned above, numerous studies have investigated the effects of physical exercise on blood redox status and muscle-damage biomarkers in trained and untrained subjects. These studies were carried out either using aerobic exercise among endurance athletes (15-18), or strenuous exercise among anaerobic-trained athletes (3, 7, 19). However, there is little information regarding the organism’s responses during aerobic and anaerobic exercises performed by athletes coming from a different specialty (20, 21).
2. Objectives
The purpose of this study is to investigate the effect of a maximal versus supra-maximal race sustained until exhaustion on lipid peroxidation, antioxidant activity and muscle-damage biomarkers in trained (i.e. long-distance and middle-distance runners) and sedentary subjects.
3. Materials and Methods
3.1. Subjects
This study is carried out on eight untrained individuals (sedentary subjects, SS) (age: 23.9 ± 4.20 yrs., body mass: 79.1 ± 19.1 kg, height: 176.6 ± 11 cm) and seventeen Tunisian national runners : nine long-distance runners (LDR) (age: 22.70 ± 3.70 yrs., body mass: 63.10 ± 7.90 kg, height: 174.90 ± 5.3 cm) and eight middle-distance runners (MDR) (age: 21 ± 1.80 yrs., body mass: 70.90 ± 8.90 kg, height: 175.40 ± 4.50 cm). The runners used to run 2 - 3 h/day, five days of the week and had a sports experience of 9 - 15 years. Before any testing, the subjects received a verbal description of the experiment, and they completed a written consent for this study. The protocol was approved by the scientific committee of the Tunisian Military Hospital.
3.2. Experimental Protocol
All tests were conducted in the Sports Medicine Centre of the Ksar-Said Physical education institute (Tunisia) on two separate occasions. The first day of testing comprised of the medical checking and the VAMEVAL test (22). This latter is a triangular event in which participants exercised to exhaustion. The test starts at 8.5 kmh-1 and the increment is 0.5 kmh-1/minute. The maximal speed reached will be defined as the maximal aerobic speed of the subject (MAS). One week after, participants performed at the same conditions, the limited-time test (23) designed to produce an important amount of lactic acid in blood a few seconds after the exercise beginning. It was also a continuous running event but with a constant supra-maximal intensity (120% of the individual MAS). All subjects were familiarized with both tests during two preliminary sessions scheduled one week before the testing period. They were instructed to not consume alcohol or beverages containing caffeine for 24 hours before the race and to not taking any vitamin supplements or changing their diet habits one week before and during the period of testing. Athletes were instructed to also mitigate their habitual training during the testing period and all subjects should refrain from all sports activity 48 hours before testing. Both contests started at 17.00 and subjects were instructed to have a light lunch 3 - 4 hours before each race. The two-exercise tests took place on a race track marked every twenty meters, the running speed was regulated by beep, and the subject stoped running when he was unable to keep the imposed rhythm and the lag relative to the cone exceeded two meters.
3.3. Blood Samples
Ten milliliters of blood were collected by venipuncture before and immediately after cessation of each race event, in tube containing EDTA and a tube without anticoagulant. The content of EDTA tube (2 mL) was used to determine hemoglobin (Hb) concentration and hematocrit (Hct) using a hematology analyzer (AU5400, Beckmann Coulter, US). These parameters were used to calculate post-exercise plasma volume changes according to the Dill and Costill method (24). The remaining blood (8 mL) was allowed to clot and serum was separated by centrifugation (3000 rpm for 15 min at 4°C). In the serum, biomarkers of muscle damage (i.e. myoglobin (Myo), creatine kinase (CK) and lactate dehydrogenase (LDH)), inflammation (i.e. IL-6 and TNF-α), lipid peroxidation (i.e. MDA) and antioxidant activity (i.e. total antioxidant status (TAS), and catalase (CAT) activity) were assessed. All analyses were performed at the Tunis military hospital laboratories of hematology and immunology.
3.4. Muscle Damage Biomarkers
Myoglobin was analyzed using a VIDAS myoglobin kit (enzyme immunoassay ELISA kit, bioMérieux, Paris, France). The principle of the assay combined a one-step sandwich immunoassay method with the final detection of the fluorescence ELFA. Absorbance was read at 540 nm and Intra-assay and inter-assay coefficients of variation were respectively 3.9 - 6.6 and 5.2% - 11.8%. CK and LDH Activities were determined with kinetic methods by using the RA-1000 Technicon analyzer and commercial kits (Biomerieux, France). The measurements were performed according to the manufacturer’s instructions. The control serum was used to check the accuracy and precision of the assay, with a maximum error of 5%. Plasma levels of IL-6 and TNF-α were determined on an IMMULITE Analyzer, using IMMULITE IL-6 and IMMULITE TNF-α kits. The measurements were performed according to the manufacturer’s instructions (EURO/DPC, Gwynedd, United Kingdom). IL-6 procedure was a sequential immunometric assay with incubation cycles (2 × 30 minutes). Intra-assay CV varied between 3.6 and 6.2%, interassay CV was 5.9% to 9.6%, and the analytical sensitivity was 5 pg/mL. TNFα procedure was also an immunometric assay but with one incubation cycle (1 × 60 minutes). Intra-assay CV was 2.6% to 3.5%, interassay CV was 4.0% to 6.5%, and the analytical sensitivity was 1.7 pg/mL.
3.5. Lipid Peroxidation and Antioxidant Activity Biomarkers
Serum MDA was performed according to a slightly modified Buege and Aust (25) method. It consists of reacting thiobarbuteric acid on MDA. Briefly, to 0.5 mL serum, 0.5 mL of 35% TCA was added. After vortex mixing, 0.5 mL Tris/HCl buffer (50 mM; pH 7.4) was added followed by further mixing and incubation at room temperature for 10 minutes; 1.0 mL of 0.75% thiobarbituric acid in 2 M Na2SO4 was added and then the mixture was heated at 100 °C for 45 minutes. After cooling, 1.0 mL of 70% TCA was added, the mixture was vortexed and then centrifuged at 950g for 10 min. The absorbance of supernatant was determined at 530 nm against a blank that contained all the reagents minus the biological sample. TBAR content was quantified as the MDA extinction molar coefficient E = 1.56 × 105 M-1cm-1, and the result was expressed in MDA nmoles/mg protein. TAS was assessed by a colorimetric determination on RA1000 using a Randox kit (TAS, Randox Laboratories, UK). The procedure is based on the capacity of plasma antioxidant substances to inhibit the oxidation of 2.2-azino-di-[3-ethylbenzthiazioline sulphonate] (ABTS) to the radical cation ABTS+ by metmyoglobin. Results were expressed in mM of Trolox equivalents. The linearity of calibration extends to 2.5 mmol/L of Trolox. The reference range for human blood plasma is given by the manufacturer as 1.30 - 1.77 mmol/L. Measurements in duplicate were used to calculate intra-assay variability. CAT was measured according to the method of Aebi (26). 0.1 mL of blood serum was pipetted into cuvette containing 1.9 mL of 50 mM phosphate buffer of pH 7.0. Reaction was started by the addition of 1.0 mL of freshly prepared 30% (v/v) hydrogen peroxide (H2O2). The rate of decomposition of H2O2 was measured spectrophotometrically from changes in absorbance at 240 nm. One unit of catalase activity was defined as the amount of catalase which was absorbed in 30 seconds at 25°C. The catalase activity was then calculated from the change in absorbance and finally expressed as U/mL.
3.6. Statistical Procedures
The SPSS version 16.0 was used for all analyses (SPSS, Chicago, IL, USA). Data were presented as mean ± standard deviation (SD). The Kolmogorov-Smirnov test was used to test the normality of all dependent variables, and was found not to differ significantly from normal values. Any potential differences in markers of muscle damage, lipid peroxidation, and antioxidant capacity between baseline and post-exercise were determined using a paired samples t-test. To evaluate any differences in the lipid peroxidation, antioxidant activity and muscle-damage biomarkers within (before vs. after-exercise) and between groups (SED, LDR and MDR), a one-way ANOVA was applied. Correlations between variables were examined by Pearson’s correlation analysis. Values at the 0.05 level were accepted as being statistically significant.
4. Results
4.1. Physical Performances
All subjects completed the two exercise tests. Data relating to the MAS noted a significant difference in LDR vs. the other two groups (P < 0.01 for all) and in MDR vs. SS (P < 0.05), whereas, at the end of the constant intensity event, a significant difference was found only between runners and sedentary subjects (LDR vs. SS, P < 0.01; MDR vs. SS, P < 0.05). No significant difference was noted between runners. (Table 1). In all subjects, a significant correlation was found at the end of the Limited-Time test between: (a) the sustained-time and age (r = -0.462; P < 0.05), and (b) the sustained-time and MAS (r = 0.549, P < 0.01).
a Values are presented as mean ± SD.
b differ significantly from LDR.
c differ significantly from MDR.
4.2. Plasma Volume Changes
A significant increase in Hct and Hb concentration were noted in LDR at the end of the VAMEVAL and the Limited-Time test (P < 0.05), while no significant differences were observed in SS and MDR (Table 2). Thus, post-exercise concentrations will be corrected for changes in plasma volume (PV) only for LDR using the method employed by Dill and Costill (24). ΔPV values were respectively -8.02 ± 4.54% and -10.03 ± 6.85%.
| Variables | VAMEVAL Test | Limited-Time Test | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Hematocrit, % | ||||
| SS | 39.71 ± 5.7 | 41 ± 4.17 | 40.01 ± 5.3 | 40.7 ± 4.34 |
| LDR | 42.43 ± 1.9 | 45.43 ± 4 b | 42.8 ± 3.2 | 45.85 ± 4.2 b |
| MDR | 40.85 ± 3.13 | 41.7 ± 3.14 | 40.79 ± 4.8 | 41.28 ± 3.68 |
| Hemoglobin, g/dL | ||||
| SS | 14.14 ± 2.19 | 14.57 ± 1.9 | 14.09 ± 2 | 14.14 ± 1.9 |
| LDR | 14.85 ± 1.34 | 15.7 ± 1.6 b | 14.12 ± 1.09 | 15.7 ± 1.6 b |
| MDR | 14 ± 1.4 | 14.4 ± 1.2 | 13.92 ± 1.7 | 14 ± 1.4 |
a Values are presented as mean ± SD.
b P < 0.05 compared to baseline.
4.3. Muscle Damage Biomarkers
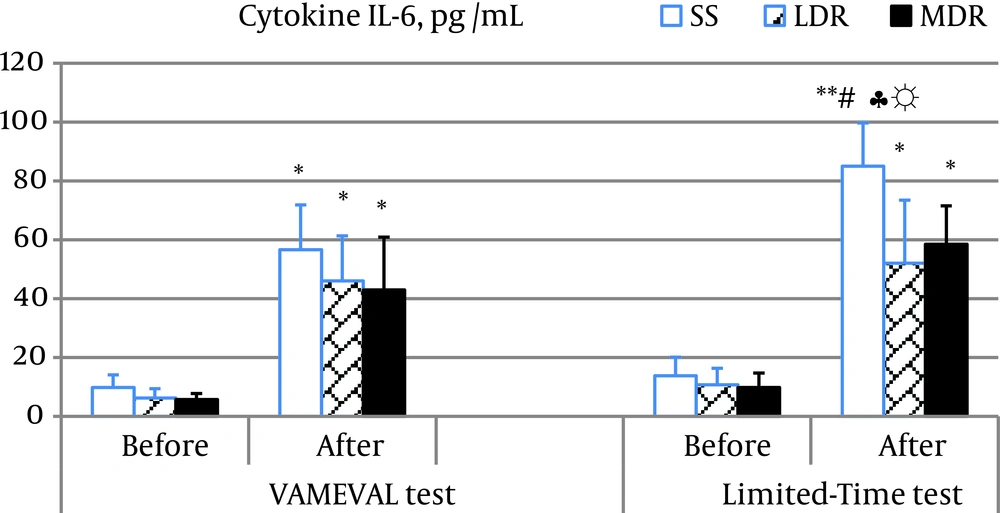
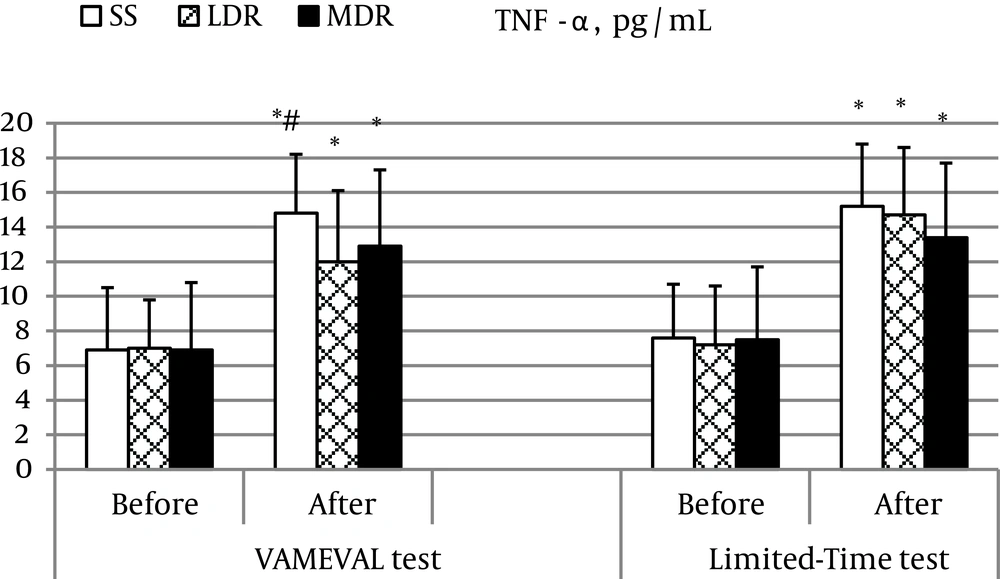
Myoglobin concentration increased significantly in all subjects after the two events (P < 0.01 for LDR after the Limited-Time test and P < 0.05 for the rest). A substantial difference was noted in MDR vs. the other two groups at the end of the constant intensity event (P < 0.05 for all) and between events (VAMEVAL vs. limited-time test) likewise in MDR (Table 3). A significant increase in CK and LDH activity was also observed in all subjects respectively at the end of the VAMEVAL and the Limited-Time test (P < 0.05 for all). A significant difference was noted after the Limited-Time test between SS and athletes (P < 0.05 for all). A noticeable enhancement in the cytokine IL-6 concentration was equally observed after the two running tests in all subjects (P < 0.05 for all) (Figure 1). The highest values were noted in SS at the end of the constant event (P < 0.05); and no differences were found between MDR and LDR. In addition, data relating to the Limited-Time test were more important than those determined after the VAMEVAL test in SS (P < 0.05). A significant correlation was observed at the end of the Limited-Time test between IL-6 concentration and CK activity (r = 0.463; P < 0.05). Significant changes in TNF-α concentration was also found after both tests in all participants (P < 0.05 for all) (Figure 2). A significant difference was observed at the end of the incremental event in LDR vs. SS (P < 0.05).
| Variables | VAMEVAL Test | Limited-Time Test | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Myoglobin, µg.L-1 | ||||
| SS | 51 ± 16 | 349 ± 106 b | 73 ± 27 | 359 ± 124 b,c |
| LDR | 76 ± 11 | 344 ± 190 b | 92 ± 31 | 365 ± 145 d,c |
| MDR | 58 ± 15 | 346 ± 243 b | 87 ± 29 | 292 ± 142 b,e |
| CK, U.L-1 | ||||
| SS | 149 ± 14 | 198 ± 16 b | 153 ± 33 | 169 ± 43 |
| LDR | 154 ± 34 | 182 ± 46 b | 162 ± 47 | 174 ± 61 |
| MDR | 150 ± 35 | 202 ± 85 b | 166 ± 45 | 177 ± 57 |
| LDH, U.L-1 | ||||
| SS | 281 ± 59 | 396 ± 82 | 301 ± 61 | 535 ± 98 d,c,f |
| LDR | 363 ± 48 | 382 ± 87 | 378 ± 66 | 501 ± 80 b |
| MDR | 372 ± 50 | 397 ± 86 | 379 ± 43 | 445 ± 64 b |
| TAS, mmoles.L-1 | ||||
| SS | 1.42 ± 0.42 | 1.38 ± 0.34 | 1.4 ± 0.61 | 1.29 ± 0.56 |
| LDR | 1.58 ± 0.28 | 1.37 ± 0.93 | 1.52 ± 0.17 | 1.19 ± 0.31 b |
| MDR | 1.76 ± 0.31 | 1.06 ± 0.27 b | 1.54 ± 0.82 | 1.62 ± 0.98 |
| Catalase activity, U.mL-1 | ||||
| SS | 2.62 ± 0.42 | 2.52 ± 0.33 | 2.53 ± 0.54 | 2.49 ± 0.33 |
| LDR | 2.95 ± 0.44 | 1.98 ± 0.11 b | 2.75 ± 0.62 | 2.88 ± 0.19 |
| MDR | 2.56 ± 0.18 | 2.45±0.48 | 2.49 ± 0.27 | 1.89 ± 0.08 |
a Values are presented as mean ± SD.
b P < 0.05.
c differ significantly to MDR.
d P < 0.01 compared to baseline.
e Differ significantly to the other event.
f Differ significantly to LDR.
4.4. Lipid Peroxidation and Antioxidant Activity Biomarkers
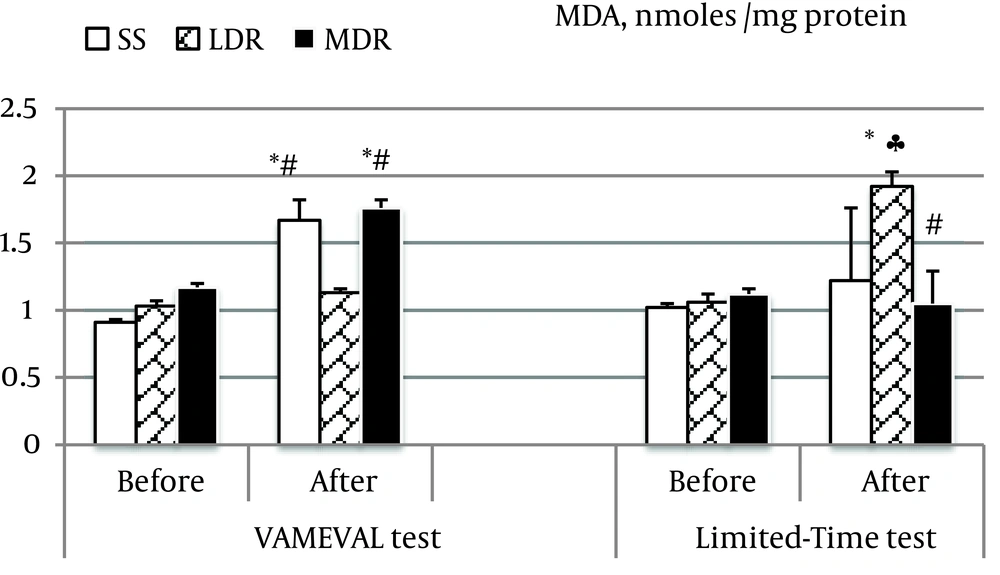
TAS decreased from rest in LDR and MDR respectively after the Limited-Time and the VAMEVAL test (P < 0.05 for all). No significant difference was found in SS and between groups or tests (Table 3). Catalase activity decreased also in LDR after the VAMEVAL test, and in MDR after the Limited-Time test (P < 0.05 for all). A substantial difference was noted between athletes (LDR vs. MDR) at the end of the Limited-Time test (P < 0.05). MDA marker of lipid peroxidation increased from rest in SS and MDR at the end of the VAMEVAL test, and in LDR after the Limited-Time test (P < 0.05 for all) (Figure 3). The highest values were notably noted, in MDR after the progressive test and in LDR at the end of the constant test. No significant difference was noted in MDR vs. SS and LDR vs. SS after the progressive and the constant event, respectively. Among the whole participants, the level of lipid peroxidation was correlated to the MAS after the two events (VAMEVAL test: r = -0.580, P < 0.01; Limited-Time test: r = 0.563, P < 0.01), and to the TAS after the constant event (r = 0.509, P < 0.01).
5. Discussion
The objective of this work was to assess the effect of two exhausting race events on lipid peroxidation, antioxidant system, and muscle damage biomarkers in athletes (i.e. long-distance and middle-distance runners) and sedentary subjects.
Results relating to baseline MDA concentrations have not shown any significant difference between groups. This agrees with the findings of some researchers noting that chronic exercise has no effect on oxidative stress level at rest (21). However, our data disagrees with the work of Marzatico et al. (20) who noted initial MDA concentrations higher among marathoners and sprinters than sedentary subjects. Our data differed also from those of Zanella et al. (27) who found initial levels significantly lower in professional footballers vs. sedentary subjects. According to Fisher-Wellman and Bloomer (1), an optimal level of RONS production appears conducive to optimal health, whereas too little or too much RONS results in impaired defense capabilities or extensive oxidative damage and inflammation, respectively.
Our findings revealed also that lipid peroxidation increased from rest in SS and MDR at the end of the VAMEVAL test and in LDR after the Limited-Time test. In runners, the recorded values are greater when the exercise testing differs from the athlete’s sportive specialty. Several studies have likewise reported increased MDA blood release after a single bouts of exercise in sedentary males (3), immediately after an exhaustive cycling exercise (15), and immediately post-endurance exercise in marathoners (20). In opposition to these findings, Niess et al. (28) did not report increased MDA concentrations following a treadmill test to exhaustion, neither at 15 minutes nor at 24 hours post-exercise in trained and untrained individuals.
The training state affects also the level of lipid peroxidation induced by strenuous physical exercise namely when the physical exercise is most close to the athlete’s sportive specialty (29). In fact, our results showed that, at the end of the incremental test, the lowest values were observed in LDR, and no difference observed in MDR vs. SS. However, after the incremental test, the lowest values were noted in MDR, and no difference noted in LDR vs. SS. These findings support many studies demonstrating a significant correlation between the training status, dietary intake, the intensity and volume of training and physical exercise damages (1). The responsible mechanisms are still poorly understood, and remain a topic of debate. Nevertheless, according to Fisher-Wellman and Bloomer (1), in addition to the increased resistance of tissues against the RONS damage (29), health benefits from regular exercise come from a reduction of basal formation of oxidants and an improvement of antioxidant defense system. CAT with superoxide dismutase and glutathione peroxidase constitute the first endogenous enzymatic defense system against oxidative stress. CAT neutralizes large amounts of H2O2 from mitochondria, and the measurement of its activity constitutes a good indicator of the oxidative stress level (1). Several studies have attempted to delineate the effect of chronic exercise on CAT activity. Nevertheless, the disagreement among the studies does not permit a clear picture to emerge. In fact, according to Lekhi et al. (16), trained subjects have a lower resting plasma catalase activity than untrained subjects. They also reported decreased serum CAT activity in trained elite cyclists immediately after a progressive test on treadmill performed to determine VO2max. on the contrary, Zembron-Lacny et al. (19) and Kyparos et al. (15) noted increased catalase activity in physical-education students exposed to a muscle-damaging resistance exercise and in well-trained rowers after a 2000-m rowing respectively.
Our results revealed also that the TAS decreased from rest in LDR and MDR respectively after the limited-time and the VAMEVAL test. No significant difference was found in SS and between groups or tests. This disagrees with the study of Diaz-Castro et al. (30) which reported increased TAS in athletes after 50 km run. Our findings have discord also with those of Schneider et al. (31) which revealed increased TAS levels in athletes at the end of 30 min of run on a treadmill at 10% below the anaerobic threshold. However, this work agrees with several studies reporting a significant decrease immediately post exercise (17). Palazzetti et al. (32) reported also decreased TAS concentrations at the end of an exhausting exercise in athletes after an overloaded training in triathlon. This decrease may reflect antioxidant compound transformations, through a process of RONS regeneration or neutralization produced during repeated metabolic phases stress.
Consistent with the latter assumption, we found that strenuous physical exercise induces muscle damage manifested by increased CK, LDH and myoglobin concentrations, and non-specific inflammatory response manifested by elevated concentrations of TNF-α and IL-6. Nevertheless, according to Pedersen et al. (33), although the acute-phase response is initiated, exercise is not followed by a fully developed systemic response. This may be because the cytokine release in response to exercise is only transient or there may be an adaptation to the cytokine response.
Compared to other studies, our findings should be viewed within the context that the performed running tests were largely comprised of concentric muscle contractions which may attenuate RONS generation from muscle injury induced by eccentric contractions. Another limit of this study exists also in the fact that lipid peroxidation, antioxidant activity and muscle damage biomarkers were assessed only immediately after effort. Some biomarkers assessed in blood may take up to two hours after the cessation of exercise to be manifested. Therefore, an experimental design incorporating all studied variables at several time points during the recovery period will be very interesting. Nevertheless, it is important to practitioners, when scheduling a training program, to take into consideration: (i) the intensity and the volume of the last training session; (ii) the training state of subjects; and (iii) the similarity between exercises and the sportive specialty of athletes. In addition, a routinely assessment of redox status and muscle damages (1-fold / year or more) should be done mainly during the competitive period or at the end of the sportive season.
In conclusion, our findings indicate that maximal and supra-maximal race events, when sustained until exhaustion, induce significant modifications in blood redox balance reflecting the production of a significant oxidative stress in trained and untrained subjects. It is also noted that the training state and the sportive specialty produced different effects on the level of the oxidative stress. MDA concentration, TAS, and CAT activity were lower in LDR and MDR at the end of the VAMEVAL and the Limited-Time test, respectively. Strenuous physical exercise also induces muscle damage manifested by increased CK, LDH and myoglobin concentrations, and a non-specific inflammatory response manifested by elevated concentrations of circulating TNF-α and IL-6. Furthermore, the athletes showed a lesser magnitude of change in the cytokine levels following exhausting exercise than non-athletes. Neither the type of the test performed nor the sportive specialty affected the recorded values.