1. Background
Physical exercise is one of the fundamental approaches for weight management (1). The mechanisms by which exercise exerts its protective effects are yet to be fully elucidated. Although most of the metabolic adaptations caused by endurance training are essential, the potential value of resistance training is also important (2-4). The American college of sports medicine (ACSM) suggested resistance training as an adult fitness program (5-8). However, the role of resistance training in the prevention of overweightness and obesity is unexplored and requires investigation. Resistance training could have a positive effect on weight control because of an increase in fat-free mass (9) and fat oxidation (10-13). Increased fat-free mass could lead to an increase in resting metabolic rate (14) and physical activity energy expenditure (15, 16). Thus, it seems that resistance training increases energy expenditure by various mechanisms (16). In 2012, it was suggested that during muscular exercise, peroxisome proliferator-activated receptor-gamma (PPAR-γ) coactivator-1α (PGC1α) expression is increased in the muscle (1, 17). Then expression of fibronectin type-III domain-containing 5 (FNDC5) is induced, which is proteolytically cleaved from muscle to form the hormone, irisin (17). In a landmark study, Boström et al. showed that irisin, a newly identified myokine, induces the browning of subcutaneous white adipose tissue by increasing the uncoupling protein-1 (UCP1), with subsequent the stimulation of oxygen consumption and thermogenesis (1, 17). This effect improved the metabolic profile and increased whole body energy expenditure (1, 17). Huh et al. (18) recently reported that irisin could improve adipose tissue metabolism and regulate muscle growth. Although the initial data on irisin from mice studies were promising, little knowledge is available from other animal species. In humans, recent studies raised numerous questions about the regulation and function of irisin and its relation to exercise (1). However, Timmons et al. (19) did not show such a response of FNDC5 mRNA in human muscle to exercise. Some other research groups have investigated the effects of exercise on irisin levels in human serum (19, 20). However, these studies had reported contradictory results. Huh et al. (18) found a significant increase in serum irisin as a response to acute exercise after moderate training of 1 week, while no result of acute exercise was seen after 8 weeks of training intervention. There was also a report about the short-term effects of exercise on irisin levels in several other studies (20, 21), but no systematic effects of exercise on circulating irisin were found (20, 22, 23). Taking into account the huge controversy in the literature about irisin and related genes and considering the Boström theory of change in phenotype from white to brown adipose tissue and, on the other hand, by considering the positive effect of resistance exercises in reduction of weight and body fat and the role of thermogenesis in this type of training compared with other types, it seems that resistance training has an effect on the phenotype of fat tissues.
2. Objectives
Based on this assumption, the question for this study is that “Can resistance exercise alter irisin levels and the expression of FNDC5 gene in the soleus muscle tissues and the UCP1 gene in the abdominal subcutaneous white adipose tissues of Sprague Dawley male rats?
3. Methods
3.1. Animals
All animal experiments were conducted in accordance with the policy of the ethics committee of the University of Kharazmi (Tehran, Iran). Sixteen Sprague Dawley male rats weighing about 200 - 220 g, 8 weeks age, were housed in special cages under normal light conditions (12-h light–dark cycles, temperature of about 23 ± 1°C, and humidity of 50 ± 3%). The animals had free access to water and were fed with pellet rodent diet ad libitum. One person was in charge of the whole study. The weight of rats was measured before and after the protocol with digital weight scale. After 2 weeks of human intervention, the rats were divided into two groups.
3.2. Exercise Protocol
This study was performed for 8 weeks, with three exercise sessions per week. During the first week, the load tied to the rats’ tails was about 50% of their body weight and was gradually increased to about 200% of their body weight in the final week. The exercise was performed on a 1.2-m long ladder in such a way that the weight was attached to the rat’s tail through a cylinder and the rat tried to climb the ladder. This exercise was performed three times, with five repetitions per week (24). The rest time between the exercise trials was 3 minutes, with an interval of 1 min between each repetition. This exercise method was adopted from some valid sources, and the influence of this type of resistance exercise on muscular preparation has been validated in previous studies (24). During this time, the control group did not do exercise.
3.3. Muscle and Subcutaneous Adipose Tissue Biopsy and Blood Samples
After 48 hours from the last exercise session (8 weeks), the animals were anesthetized intraperitoneally with a mixture of xylazine (3 - 5 mg/kg of body weight) and ketamine (30 - 50 mg/kg of body weight). From each animal, 3 mL of blood was collected from the right ventricle and immediately transferred to an EDTA tube. The blood samples were centrifuged for 15 minutes at 1,000 × g for separation of plasma. All the collected plasma samples were stored in a deep freezer (-80°C) for subsequent measurements. After collecting the blood samples, the abdominal part of the rats was opened and a portion of the subcutaneous abdominal adipose tissue was excised and washed in ice-cold saline. In addition, the soleus muscle tissues were cut out and, after measuring the weight, they were stored in a freezer (-80°C) for further usage.
3.4. Measurement of Plasma Irisin Levels
Using Soluble FNDC5 (Human, Mouse, Rat) ELISA Kit provided by the Canadian Company of Phoenix (Cat. No: EK-067-53), the irisin levels in the plasma samples were determined.
3.5. Quantitative Real-Time PCR Analysis
All measurements were performed in duplicate. For extraction of RNA, 50 mg of the frozen adipose or muscle tissue was homogenized and total RNA was isolated by the RNA-Plus kit (Cinna Gen., Iran), according to the manufacturer’s instructions. Then, the RNA solution was extracted and decontaminated from any DNA and RNA destructive enzymes, using RNase-free DNaseI (Fermentas, Germany). From each sample, 2 µg of RNA was used for synthesizing the first cDNA using the cDNA Synthesis Kit (Vivantis) utilizing the oligo-dT primer. RT-PCR was performed using 2 µg of cDNA and 5 pmol of each primer in a total volume of 20 µg PCR reaction mixture. Real-time PCR was conducted in a thermal cycler (Biorad., USA), as suggested by the protocol (TaKaRa). The PCR mixture contained 10 µg of Rotor-Gene SYBR Green PCR Master Mix (TaKaRa), 3 pmol of each primer, and 25 ng of cDNA for each reaction in a final volume of 20 µg. Relative mRNA concentrations were calculated from take-off point of reactions using the software provided by the manufacturer and normalized to β-actin expression level in the same samples. Data were assessed and reported according to the ΔΔCt method. The list of primers is shown in Table 1.
| Genes | Primers | Melting Temperature (°C) |
|---|---|---|
| FNDC5 | ||
| F | 5′-GTCTCCCACCACCATCTT-3′ | 63 |
| R | 5′-TCTGTCTCTGAGTGTAGCCTTAGC-3′ | 63 |
| Β-actin | ||
| F | 5′GGAGAAGATTTGGCACCACAC-3′ | 54 |
| R | 5′-GGATGGCTACGTACATGGCTG-3′ | 56 |
| UCP1 | ||
| F | 5′-GATCCTGGAACGTCATCATGTTTGTG-3′ | 60 |
| R | 5′-CCCAATGGTTAGCATCCCTTTC-3′ | 66 |
aF and R, forward and reverse primer respectively.
3.6. Statistical Analysis
All statistical results were expressed as mean ± SEM (standard error of mean). Independent t-test was used to compare groups. SPSS (version 19) software was used for all statistical analyses. P < 0.05 were considered to be statistically significant.
4. Results
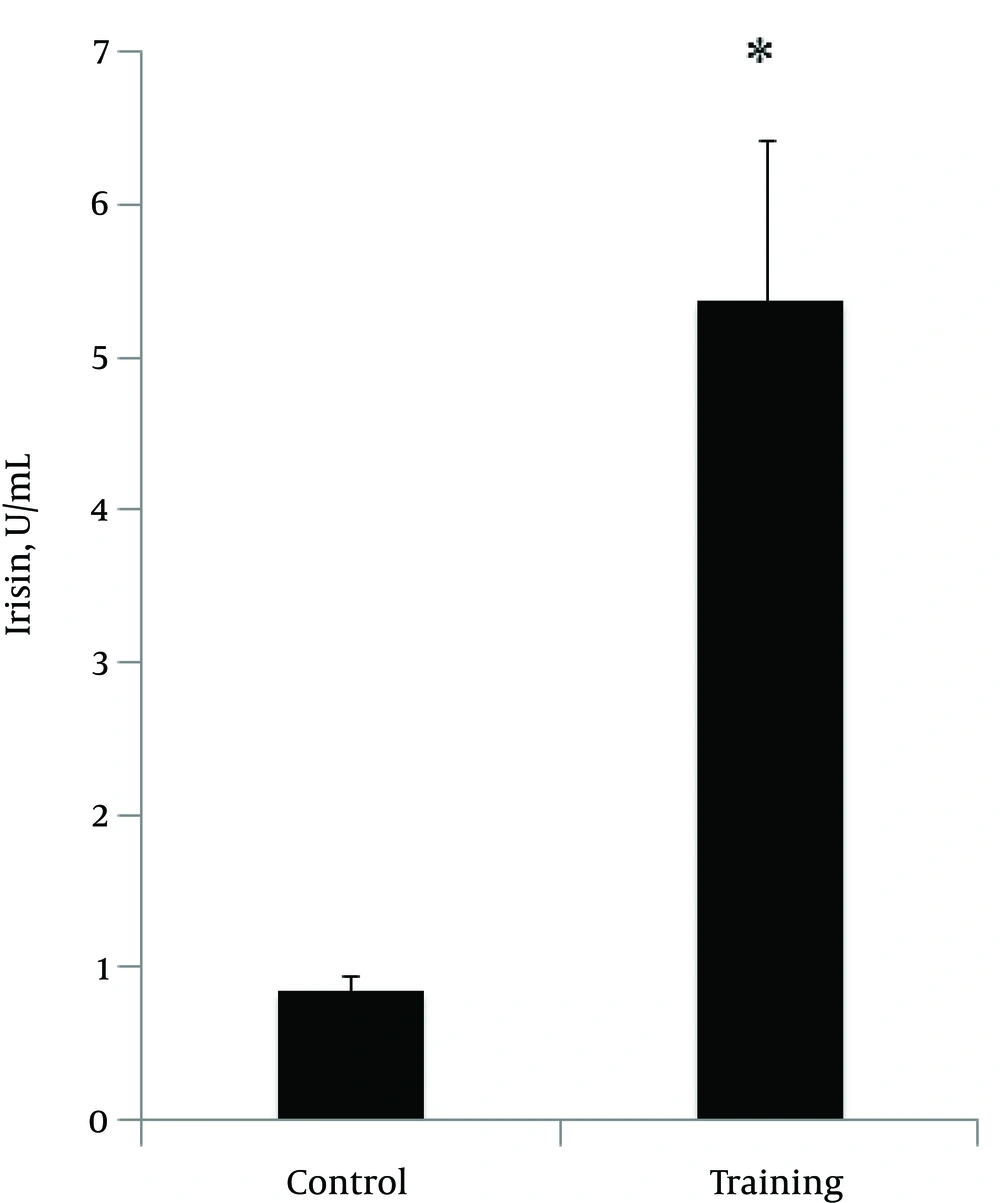
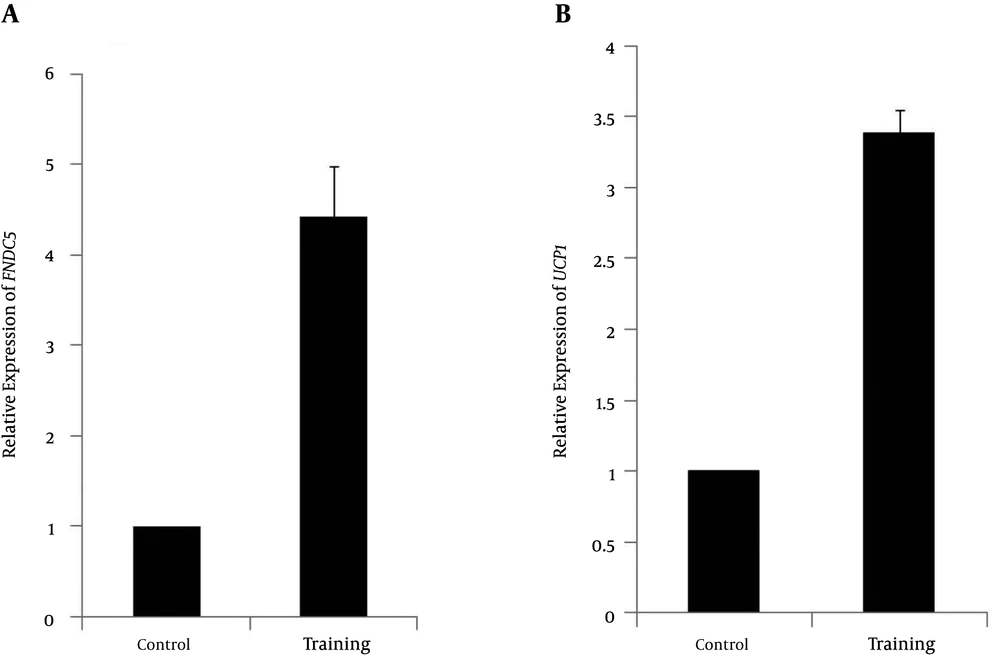
The normality of the distribution was analyzed using Shapiro-Wilk test (P = 0.254, t = 1.18). Data showed that plasma irisin levels were significantly higher in the resistance-trained group than those in the control group (P < 0.001, t = 4.48) (Figure 1). Soleus FNDC5 expression was also significantly increased (P < 0.001, t = 6.18) in the trained rats (Figure 2A). In addition, abdominal subcutaneous white adipose tissue UCP1 expression was significantly (P < 0.001, t = 14.26) higher in the trained rats (Figure 2B). No significant difference in the rats’ weights was observed before (P = 0.254, t = 1.18) and after exercise (P = 0.662, t = 0.44). However, the mean weight gain of the exercise group was lower, although not significantly different between the groups (P = 0.096, t = 1.72). The soleus muscle’s weight showed a significant increase after the exercise (P < 0.000, t = 5.0) (Table 2).
A, The changes in relative expression of FNDC5 of mRNA genes with respect to β actin gene in exercise (4.43 ± 0.55) and control groups; B, The changes in relative expression of UCP1 of mRNA genes with respect to β actin gene in exercise (3.39 ± 0.16) and control groups; *significant in P < 0.001.
| Variable | Exercise Group | Control Group | t | P value |
|---|---|---|---|---|
| Weight before exercise, gr | 214.1 ± 9.73 | 208.5 ± 7.97 | 1.18 | 0.25 |
| Weight after exercise, gr | 282 ± 9.9 | 301.1 ± 12.5 | -0.4 | 0.66 |
| Weight gain (mean), gr | 68.7± 6.52 | 92.5± 12.66 | 1.72 | 0.09 |
| Soleus weight, gr | 0.05 ± 0.002 | 0.025 ± 0.004 | -5 | 0 |
aValues are expressed as mean ± SEM in each group (n = 8).
5. Discussion
The present study was intended to address whether 8 weeks of resistance training modulated plasma irisin levels and expression of muscle FNDC5 and subcutaneous adipose tissue UCP1 genes. Our data revealed a significant increase in plasma irisin levels (Figure 1) and an increase in mRNA expression of FNDC5 and UCP1 genes after 8 weeks of resistance training (Figure 2A and 2B). These results demonstrate that 8 weeks of resistance exercise probably results in the release of irisin hormone. Therefore, it seems that resistance exercises are stimulants for this myokine and related genes (25). In a previous study, we showed that one session of resistance training increased plasma irisin protein levels and expression of soleus muscle FNDC5 and abdominal subcutaneous adipose tissue UCP1 genes (25). Exercise training is well known for its beneficial effects on metabolism (25). In the past few decades, several animal studies have investigated whether exercise has beneficial effects on BAT activity (17, 26-29). Studies in rodents showed that exercise has stimulating effects on brown adipose tissue (17, 26-29). Another study showed that a low level of exercise training in rats is effective for the metabolic response under cold exposure in brown adipose tissue (26). Xu et al. (28) showed that exercise could result in a twofold increased recruitment of adipogenic progenitor cells in the inter-scapular expression of BAT and UCP1 in mice. Besides these stimulating effects, they also found an increased thermogenic expression in visceral adipose tissue, including increased UCP1 levels (twofold). De Matteis et al. (30) demonstrated the occurrence of browning of visceral cells of rats after 1 week of exercise. An eightfold increase was observed in the number of brown cells in the exercise rats compared with controls (30). A recent study by Boström et al. showed that endurance exercise predominantly causes browning of subcutaneous white adipose tissue (17). They observed that mice that overexpressed the transcriptional co-activator PGC1-α showed increased browning in inguinal white adipose tissue (17). In addition, as exercise also increases PGC1-α, these investigators considered the effect of endurance exercise on markers of browning and observed similar effects. Furthermore, they found that the irisin precursor FNDC5 induced browning in primary subcutaneous white adipose tissue, which was demonstrated by increased UCP1 mRNA expression (7 to 500-fold) and upregulation of thermogenic genes (UCP1). In FNDC5-overexpressed mice, plasma irisin levels were increased by three to fourfold, which increased UCP1 mRNA expression by 13-fold in subcutaneous white adipose tissue (17, 29). In addition, the same exercise methods were used in C557BL/6 mice, which showed increased irisin levels leading to improved glucose tolerance, decreased fasting insulin, increased oxygen consumption, and reduced body weight (17, 29). All these results indicate the possible beneficial effects of exercise on the recruitment of brown cells within subcutaneous white adipose tissue and, consequently, the potential of irisin to induce a healthier metabolic phenotype (29). Our results showed that after 8 weeks of resistance training, irisin levels and mRNA expression of UCP1 in subcutaneous white adipose tissue and muscle FNDC5 increased. Similarly, Prestes et al. (2015) (31) showed that high-intensity resistance training increased irisin levels after 16 weeks of training in sedentary elderly women. Increases in serum irisin, FNDC5, or PGC1-α after chronic exercise have been reported in mice and humans (1, 17). However, Timmons et al. failed to detect any significant effects of exercise on FNDC5 muscle expression in a larger cohort, despite improvements in physical fitness and insulin sensitivity, observing about 30% up regulation of FNDC5 gene only in the skeletal muscle of highly active elderly subjects (19). Lecker et al. have shown that FNDC5 expression was higher in the group of individuals with a higher aerobic performance (23, 32), and Huh et al. (21) reported a mild increase in circulating irisin levels 30 min after sprint exercise in 15 moderately trained healthy young men, but failed to detect an effect of 8 weeks of exercise training intervention. In contrast to our results, Kurdiova et al. (23) showed that exercise training failed to affect circulating irisin levels as well as muscle FNDC5 expression. Moreover, muscle FNDC5 mRNA expression and circulating irisin levels (60 minutes post-exercise) were unaffected by an acute bout of exercise in both sedentary and trained individuals, indicating that muscle contraction is perhaps not the primary switch of FNDC5/irisin production. The large inconsistency could be related to the differences in the study design and populations. Besides the species differences, these discrepancies may be related to physiological and technical differences between the studies. The different types (sprinting, cycling, or swimming; endurance or resistance) and intensities of exercise would have diverse influence on muscle metabolism and disruption and, in turn, on irisin concentrations (1). Another factor is the timing of irisin measurement after exercise; since studies have measured irisin levels at different time points, the possibility exists that irisin increases for a short period post-exercise, after which it returns to baseline concentrations (1). There may also be a mechanism for irisin uptake or clearance from circulation, which has not been assessed yet.
5.1. Conclusion
It appears that irisin increases in adaptation to resistance exercise in rats, and its production leads to the following sequence: resistance exercise stimulates FNCD5 gene expression, causing increased irisin levels. Then, it seems that irisin causes the browning of subcutaneous white adipose tissue and increases energy expenditure via thermogenesis, independent of exercise or food intake in rats. In conclusion, future studies must investigate the direct effects of exercise on browning of subcutaneous white adipose tissue and should focus on various types of training exercise in rodents and humans.